Fetal Bovine Serum (FBS) – What You Need to Know
- How to Handle Serum in the Lab
- Geography, Pathogens, and FBS Sourcing
- International Testing and Regulatory Practices
- Traceability and Documentation in the Serum Industry
- Economics 101: Global Factors that Affect FBS Price
- Related Products
A lab commodity whose quality may often be taken for granted, serum can make—or break—the outcome of all of your cell-based assays and cell culture applications. These 5 key subjects will provide you with a strong educational foundation in the use of sera for cell culture.
Biologists who culture cells or tissue know that serum products including fetal bovine serum, or FBS, (also sometimes called FCS, or fetal calf serum) constitute an essential component of culture media for most applications. Serum is often one of the most abundant constituents of cell culture media (typically 2-15% by volume), so its quality can have significant impact on culture success.
FBS in particular is the mostly widely-used media supplement for the culture of mammalian cells due to its ready availability, along with its rich supply of growth factors that promote the development and expansion of cells in vitro. Serum also acts as a buffer to the cell culture system against a variety of toxic effects that can disrupt cell growth such as pH change, proteolytic activity, or the presence of endotoxin.
We make it simple to determine which FBS will work best for your application and budget by dividing our FBS portfolio into three distinct categories - Classic, Premier, and Select. Our FBS product categories are based on the relevant parameters for your application:
- serum tested for the absence of specific contaminants
- γ-irradiated, nonirradiated, and heat-inactivated serum
- geographic origin
- suitability for stem cell and cardiomyocyte culture
- sterile-filtered or unfiltered serum
How to Handle Serum in the Lab
Anyone who has ever ordered serum for the lab knows that delivery timing is important, as bottles will typically arrive frozen from the supplier. This is because the activity of critical macromolecular factors in serum are best protected by storing serum at -5 to -20 °C, and maintaining serum in the frozen state until use will also reduce the incidence of events like the breakdown of lipids.
Serum should be thawed in a 30-37 °C water bath or incubator. It is important that the bottle of serum is periodically agitated to reduce the salt and protein gradients that can form in the serum during thawing. These precipitates are not toxic to cell cultures, but they can influence the reproducibility of cellular data. Do not exceed temperatures of 37 °C, which can compromise serum performance by accelerating the degradation of critical serum components.
Serum can be sensitive to environmental conditions beyond temperature. For example, high intensity/high energy wavelengths of light may degrade the activity of growth factors, which is important to keep in mind when using serum near UV lighting intended to promote sterility in some biosafety cabinets. Typical laboratory lighting conditions will have little to no effect on serum integrity or performance.
Physicians may interpret a cloudy patient sample to be indicative of lipemia, or excess fats in the blood. The same may be true of that cloudy serum in the lab — if bacterial contamination can be ruled out by culturing a sample, cloudy serum is most likely explained by the precipitation of lipids and lipoproteins.
Cloudy serum can be minimized by aliquoting bulk stock into single-use tubes to avoid repeated freeze-thaw cycles. Although the presence of the clotting protein fibrin is sometimes blamed for serum cloudiness, serum is prepared from the post-coagulation fraction of blood, and therefore would not typically contain significant amounts of fibrin or other clotting factors.
Good practices such as aliquoting serum into single use tubes for frozen storage reduces the potential for repeated exposure to contaminants. Aliquoting also helps to prevent cloudiness that can arise from the breakdown of lipids and lipid-associated macromolecules as a consequence of repeated freeze-thaw cycles.
It’s also useful to realize that lipid content contributing to cloudiness in serum can vary significantly among species. For example (and perhaps not surprisingly), porcine serum is higher in lipid content than its bovine counterpart. So, serum from diverse biological sources requires unique handling and additional processing steps at the manufacturing facility to prepare the final product for use in the lab. Suppliers that offer sera from multiple species will therefore have differential protocols in place for processing raw materials in order to provide a consistent, appropriate serum product to the end user, regardless of lipid biology.
Geography, Pathogens, and FBS Sourcing
Because serum is a biological product, it is subject to variations that arise from the health of the source animals, which is in turn influenced by dietary, environmental, and global disease factors. One of the most important attributes knowledgeable serum product users therefore seek is the country of origin, or source.
Although the incidence and impact of various species-specific pathogens such as bovine spongiform encephalopathy (BSE) on serum are still being studied, the USDA maintains lists of countries approved for importation of serum that are based in part on up-to-date pathogen incidence data. Serum consumers should therefore always expect suppliers to provide geographical source information for their sera and other products of animal origin.
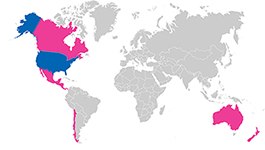
The USDA maintains a list of countries approved for importation of serum into the U.S. (guidelines revised annually). This differs from U.S.-origin serum, which is collected from facilities in the U.S. that undergo continuous inspection by the USDA.
International Testing and Regulatory Practices
Reputable suppliers will typically employ consensus testing methods specified by the serum industry to ensure that serum supplied to laboratories meets standards for levels of common contaminants that may impact its performance in cell culture. Some common tests are:
Standards for the testing of general serum properties and components have also been established to enable consistent evaluation for osmolality, total protein, hemoglobin, and general performance in culture.
Traceability and Documentation in the Serum Industry
While “origin” indicates the country in which serum was collected, “traceability” refers to how well the journey of a serum product — from collection at site of origin through manufacturing and distribution — has been documented.
Serum delivered frozen to a processing facility is thawed and tested for the presence of bacteria, virus, endotoxin, and for hemoglobin content. The best quality serum is then filtered through a sequence of membrane filters, terminating with a 0.1 µm pore filtration under aseptic conditions.
After filtration, aseptic procedure is maintained as serum is dispensed into sterile bottles, quickly frozen to -20 °C and quarantined until all lots have been shown to meet or exceed quality control standards. A certificate of analysis for each lot should always be available upon request from the supplier.
Traceability for biologics is critical to ensure that label descriptions are an accurate representation of product history and contents. Complete documentation ensures that both the customer and regulatory agencies can audit the processing of serum products, and can uncover whether suppliers are engaging in serum pooling to offset local supplier cost fluctuations — a practice which can compromise performance.
Economics 101: Global Factors that Affect FBS Price
Serum is a commodity product for which price varies among countries of origin. For example, the cost of serum originating from Australia, New Zealand, and the United States may be higher than serum from other countries.
The OIE’s (World Organization for Animal Health) current Geographical Risk Assessment rating for these countries and for the United States is high because they continue to be free of BSE and FMD (foot and mouth disease). For Australia and New Zealand, this is in part due to geographical isolation. Strict agricultural regulatory practices and increased demand for sera from these countries can drive up price.
The availability of FBS and other animal sera is inherently subject to fluctuations in global animal disease states, climate, and food and water supply. When there are global weather events, it can often be difficult for farmers to move their cows along the process to obtaining serum. Recently, herds have been at record lows and farmers are rebuilding their herds, decreasing the amount of serum on the market. These factors make industry-wide serum pricing sensitive in ways that pricing for non-biological reagents is not.
And last — but certainly not least — as cell culture becomes more prevalent in more countries, particularly in the pharmaceutical sector, serum pricing is sure to reflect the changes in supply this demand may precipitate. These conditions cause instability in the supply and demand of FBS, leading to global price fluctuations for sera.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?