The Derivatization and Analysis of Amino Acids by GC-MS
Introduction
Typically, high-performance liquid chromatography (HPLC) is used for the analysis of amino acids. However, GC can also be used, and in some cases availability of instrumentation or operation costs can make it a better choice. The polar nature of amino acids requires derivatization prior to GC analysis. The goal of derivatization is to make an analyte more volatile, less reactive, and thus improve its chromatographic behavior. In the case of amino acids, derivatization replaces active hydrogens on OH, NH2, and SH polar functional groups with a nonpolar moiety.
Section Overview
Silylation is a very common derivatization technique, and is useful for a wide variety of compounds. The main disadvantage of this method is its sensitivity to moisture. The presence of moisture results in poor reaction yield and instability of the derivatized analytes. For this study, we evaluated the use of the silylation reagent N-tert-butyldimethylsilyl- N-methyltrifluoroacetamide (MTBSTFA) for the derivatization of amino acids. MTBSTFA, forms tert-butyl dimethylsilyl (TBDMS) derivatives when reacted with polar functional groups containing an active hydrogen:
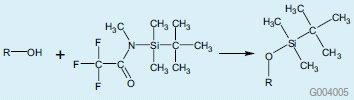
Figure 1. Structure of MTBSTFA
MTBSTFA derivatives are more stable and less moisture sensitive than those formed using lower molecular weight reagents such as N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (1).
Experimental
A 50 μL aliquot of a solution containing a mix of L-amino acids at 91 μg/mL in 0.1 N HCl was dried, and 100 μL of neat MTBSTFA, followed by 100 μL of acetonitrile, were added. The mixture was heated at 100 °C for 4 hours. The sample was then neutralized with sodium bicarbonate and subjected to GC-MS analysis on a 20 m x 0.18 mm I.D. x 0.18 μm SLB™-5ms capillary column.
Results
A chromatogram of the TBDMS derivatives of the amino acids is presented in Figure 2. Spectral data obtained from the peaks helped in identification of the amino acid derivatives. Replacement of an active hydrogen with a TBDMS group adds 114 to the molecular weight. Electron impact spectra (2) of these derivatives contains typical fragments corresponding to the molecular weight of the derivative less CH3 (M-15), C4H9 (M-57), C4H9 + CO (M-85), and CO-O-TBDMS (M-159). Figure 3 shows an example of this fragmentation pattern in the spectrum of TBDMS-valine.
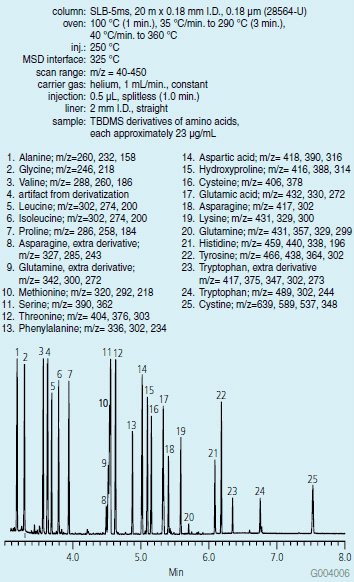
Figure 2. GC-MS Analysis of Amino Acid Derivatives on the SLB-5ms (28564-U)
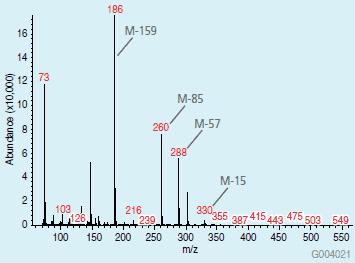
Figure 3. Mass Spectrum of TBDMS Derivative of Valine (MW of the derivative = 345)
Under the reaction conditions used, the majority of the amino acids produced one derivative, with active hydrogens on hydroxyl, amine, and thiol groups (in the case of cysteine) replaced by TBDMS. Some amino acids produced multiple derivatives, specifically asparagine, glutamine, and tryptophan. In the case of these amino acids, a modification in the reaction conditions, such as, lowering the temperature or changing the reaction time, may prevent this from occurring (3). For example, increasing the reaction time from 2 to 4 hours resulted in an increased response of the fully derivatized form of tryptophan.
While TBDMS derivatives are more stable than traditional TMS derivatives, their higher molecular weights result in longer elution times during GC analysis. To balance this, the separation was done on a short, narrow bore capillary column. A starting temperature no higher than 100 °C was necessary to maintain resolution of the glycine derivative peak from the solvent. A quick ramp to 360 °C after the elution of the cystine derivative was performed to ensure the column was clean for subsequent analyses.
Conclusion
This study demonstrates that with the proper use of derivatization reagents such as MTBSTFA, amino acids can be analyzed by GC-MS. The reaction conditions may have to be “tweaked” to produce maximum response of the derivatives of interest. The derivatives produce characteristic fragments, allowing for easy identification by MS. To reduce the overall GC analysis time of these derivatives, a short, narrow bore column such as the 20 m x 0.18 mm I.D. x 0.18 μm SLB-5ms is recommended.
References
To continue reading please sign in or create an account.
Don't Have An Account?