Prestige Antibodies® Powered by Atlas Antibodies in Western Blot Application
Western Blot
Western Blot (WB) is considered to be the most sensitive, simple, and unequivocal immunodetective application available. This is due to the high amount of information obtained upon performance, such as target molecular weight and quantitative, as well as qualitative, information of the protein of interest.
The Human Protein Atlas
The Human Protein Atlas (HPA) project 1,2 has been set up to allow for a systematic exploration of the human proteome using Antibody-Based Proteomics. This is accomplished by combining high-throughput generation of Prestige Antibodies® with protein profiling in a multitude of human tissues and cells. The standard evaluation of the Prestige Antibodies® is immunochemical staining (IHC, ICC), but all antibodies are also tested in WB and Immunofluorescence (IF). The resulting IHC, WB, ICC, and IF images are publicly available on the Human Protein Atlas web portal.3 Each year, protein expression and localization data of approximately 2,500 new proteins are added to the portal. By 2015, a first draft of the localization of the full human proteome is expected to be available.
The Prestige Antibodies® are tested in WB (Figure 1) in a standardized sample set-up using total protein lysates. Testing is done in a single attempt and the resulting blot images are shown on the HPA portal. For the majority of the Prestige Antibodies®, the protein sample set-up in HPA is lysate of liver tissue, tonsil tissue, urinary bladder transitional cell carcinoma cell line RT-4, glioblastoma cell line U-251MG, and IgG/HSA-depleted human plasma. The entire blot with all five sample lanes is presented, regardless of whether the lanes show single bands, no bands or multiple bands.
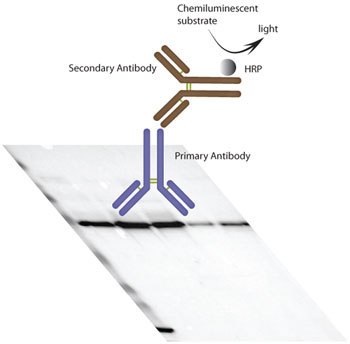
Figure 1.Schematic figure of the WB procedure used within the Human Protein Atlas project. By electrophoresis, proteins are separated according to molecular weight. After electro-transfer and immobilization on membrane, Prestige Antibodies® are used as primary antibodies for detection of target proteins. Antibody binding is visualized by chemiluminescence detection in a CCD-camera system using a horse radish peroxidase (HRP) labelled secondary antibody.
Influences of Biological Features on WB Detection
Failure in detection, i.e. no or only background detection, may not necessarily be related to a technical problem, but rather to biological features of the analyzed protein. For example, the target protein may be solely expressed during short processes or stages in the cell or only present in specific cells and tissues. The protein may also be present in amounts too low to detect.
Why Does the Actual Band Size Differ from the Predicted?
The migration of a protein through a gel is affected by other factors than the size of the protein. Such factors include:
- Splice variants, one gene can generate proteins of different sizes due to alternative splicing
- Post-translational modifications may increase the protein molecular weight
- Activation through post-translational cleavage of pro-proteins may decrease the protein molecular weight
- Presence of dimers and multimers may increase the protein molecular weight
Score System in the Human Protein Atlas
The performance of the Prestige Antibodies® in WB is validated in HPA by a score system, resulting in supportive, uncertain, and not supportive assignments. Supportive results correspond to achieved bands of predictive size with, or without, additional bands present (Figure 2 and 3). Uncertain results correspond to empty blots (no bands) or to single bands differing more than +/- 20% from predicted sizes in kDa. Finally, not supportive results refer to blots with bands not corresponding to predicted sizes or to weak bands of predicted sizes, but with additional bands of higher intensity also present.
On the HPA, WB images scored as supportive and uncertain are shown, providing free assessment of the data. WB images scored not supportive are not presented.
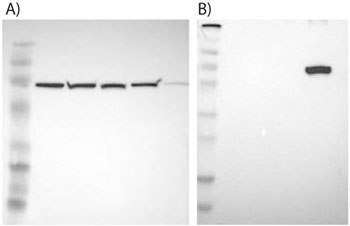
Figure 2.Examples of supportive WB images showing single bands corresponding to predicted sizes in kDa (+/-20%).
A) HPA000898, Anti-HSPA9, target weight: 74 kDa. Lane 1: Marker [kDa]: 206,113,82,49,32,26,17.8; Lane 2: RT-4; Lane 3: U-251MG sp; Lane 4: A-431; Lane 5: Liver; Lane 6: Tonsil.
B) HPA007292, Anti-SLC27A5, target weight: 75, 67 kDa. Lane 1: Marker [kDa]: 230,130,95,72,56,36,28,17,11; Lane 2: RT-4; Lane 3: U-251MG sp; Lane 4: Human Plasma; Lane 5: Liver; Lane 6: Tonsil.
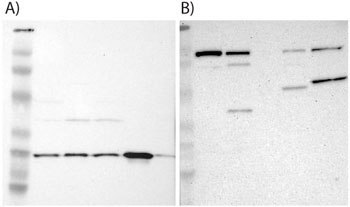
Figure 3.Examples of supportive WB images showing bands of predicted sizes in kDa (+/-20%) with additional bands present.
A) HPA002877, Anti-PGRMC1, target weight: 22 kDa. Lane 1: Marker [kDa]: 206,113,82,49,32,26,17.8; Lane 2: RT-4; Lane 3: U-251MG sp; Lane 4: A-431; Lane 5: Liver; Lane 6: Tonsil.
B) HPA004125, Anti-MARS, target weight: 101, 49 kDa. Lane 1: Marker [kDa]: 230,110,82,49.3,32.2,25.5,17.6; Lane 2: RT-4; Lane 3: U-251MG sp; Lane 4: Human Plasma; Lane 5: Liver; Lane 6: Tonsil.
WB in Over-expressed Lysates
Ongoing testing in over-expressed lysates (Origene Technologies) for antibodies previously scored as uncertain or not supportive using total protein lysates, results in a supportive score for 90% of the analyses.
Immunoblotting Protocol
Electrophoresis and Blotting
Sample Preparation
Protein samples (selected tissue lysates, cell lysates or over-expression lysates) are mixed with Laemmli buffer (to a final loading concentration of 2% SDS, 10% glycerol, 0.002% bromophenol blue, 0.0625 M TrisHCl), supplemented with DTT to a final concentration of 50 mM, and incubated in 95 °C for 5 minutes.
Procedure
- Protein samples are loaded onto Criterion TGX Precast Gels, 4–20% polyacrylamide. The electrophoresis is run according to manufacturer's protocols.
- The proteins are transferred from the gels to PVDF membranes through semi-dry transfer using Trans-Blot® Turbo transfer system (Bio-Rad) according to manufacturer's protocol.
Immunodetection
All incubation and wash steps are performed at room temperature and with agitation.
Procedure
- Dried membranes from previous steps are activated in methanol for 20 seconds. To prevent non-specific background binding of the primary and/or secondary antibodies to the membrane, membranes are blocked in milk-based blocking buffer (5% (w/v) non-fat dried milk in TBS with 0.1% (v/v) TWEEN® 20) for 45 minutes.
- The primary antibody is diluted in blocking buffer and incubated with the blocked membranes for 1 hour.
NOTE: The recommended working dilution of the primary antibody is to be considered as a guideline only. Optimal dilution must be determined by the user.
- To remove residual primary antibody, the membranes are washed 3 x 5 minutes in TBST (TBS with 0.1% (v/v) TWEEN® 20).
- The secondary antibody (for monoclonal antibodies: HRP-conjugated Goat Anti-Mouse Immunoglobulin; for polyclonal antibodies: HRP-conjugated Swine Anti-Rabbit Immunoglobulin) is diluted 1:3000 in blocking buffer and incubated with the membranes for 30 minutes.
- To remove residual secondary antibody, the membranes are washed 4 x 5 minutes in TBST.
- The membranes are incubated with detection reagent for 1 minute.
- The image is captured using a CCD camera.
Summary
- 60% of the Prestige Antibodies® are recommended for the Western Blot application and scored as supportive on HPA.
- The Prestige Antibodies® have been analyzed in a single WB attempt using a standardized immunoblotting protocol and sample set-up consisting of four total protein lysates and human plasma.
- The entire Western Blots are presented on the Human Protein Atlas portal, allowing for free assessment of the data.
- Each Prestige Antibody approved for WB use is also approved for use in IHC. More than 500 IHC images per antibody show protein expression and localization in a large variety of different normal and cancer tissues. All IHC data is publically available on the Human Protein Atlas portal.
References
To continue reading please sign in or create an account.
Don't Have An Account?