Performing a Separation with Sephadex®
Desalting and buffer exchange can take less than 5 min/sample with greater than 95% recovery for most proteins.
To prevent possible ionic interactions the presence of a low salt concentration (25 mM sodium chloride) is recommended during desalting and in the final sample buffer. Volatile buffers such as 100 mM ammonium acetate or 100 mM ammonium hydrogen carbonate can be used if it is necessary to avoid the presence of sodium chloride.
The sample should be fully dissolved. Centrifuge or filter to remove particulate material (Appendix 3). Always use degassed buffers to avoid introducing air into the column.
Sample concentration up to 70 mg/mL protein should not influence the separation when using normal aqueous buffers.
If possible, use a chromatography system with both a UV and a conductivity monitor to facilitate optimization of the sample loading. The elution of the protein peak at A280 and the appearance of the salt peak can be followed exactly and different separations can be easily compared as shown in Figure 5.6.
If conductivity cannot be monitored and recovery of completely desalted sample is the major requirement, apply sample volumes of between 15% and 20% of the total column volume.
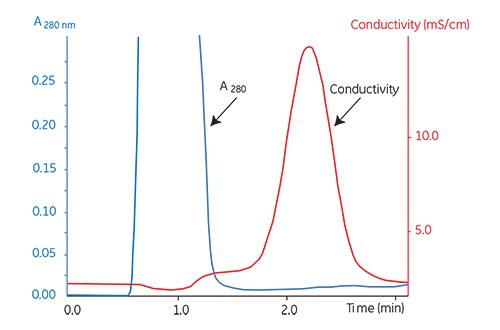
Figure 5.6.Buffer exchange of mouse plasma on HiPrep™ 26/10 Desalting.
General considerations
Small-scale desalting of samples
Or sample volumes ranging from 0.2 to 2.5 mL, it is possible to run multiple samples in parallel with PD-10 Desalting, PD MidiTrap™ G-25, and PD MiniTrap™ G-25 columns. Two different protocols are available for these columns: one for manual use on the laboratory bench and one for use together with a standard centrifuge in combination with a Spin Adapter. For smaller proteins (Mr > 700), PD MiniTrap™ G-10 and PD MidiTrap™ G-10 columns may be used.
For smaller sample volumes in the range of 70 to 130 μL, multiple samples can be run on PD SpinTrap™ G-25 spin columns together with a microcentrifuge or PD MultiTrap™ G-25 96-well plate using centrifugation for extraction. Although possible to perform, using PD MultiTrap™ G-25 with vacuum is not recommended due to reduced reproducibility compared with operation using centrifugation.
Desalting larger sample volumes using HiTrap® and HiPrep™ columns
Connect up to three HiTrap® Desalting columns in series to increase the sample volume capacity. For example, two columns allow a sample volume of 3 mL, and three columns allow a sample volume of 4.5 mL.
Connect up to four HiPrep™ 26/10 Desalting columns in series to increase the sample volume capacity. For example, two columns allow a sample volume of 30 mL, and four columns allow a sample volume of 60 mL. Even with four columns in series, the sample can be processed in 20 to 30 min without back pressure problems.
Buffer preparation
For substances carrying charged groups, an eluent containing a buffer salt is recommended. A salt concentration of at least 150 mM is recommended to prevent possible ionic interactions with the chromatography medium. Sodium chloride is often used for this purpose. Often a buffer with 25 to 50 mM concentration of the buffering substance is sufficient. At salt concentrations above 1 M, hydrophobic substances can be retarded or can bind to the chromatography medium.
At even higher salt concentrations, > 1.5 M ammonium sulfate, the column packing shrinks.
Sample preparation
Sample concentration does not influence the separation as long as the viscosity does not differ by more than a factor of 1.5 from that of the buffer used. This corresponds to a maximum concentration of 70 mg/mL for proteins, when normal, aqueous buffers are used. The sample should be fully solubilized. Centrifuge or filter (0.45 μM filter) immediately before loading to remove particulate material if necessary.
Buffer exchange
Protein solubility often depends on pH and/or ionic strength (salt concentration), and the exchange of buffer can therefore result in precipitation of the protein. Also, protein activity can be lost if the change of pH takes it outside of the range where the protein is active. Samples that have been obtained after purification will usually be free from particles, unless the purified protein or a contaminant has been aggregated.
The protocols in the following sections describe desalting and buffer exchange using different formats of prepacked columns.
HiTrap® Desalting columns
Figure 5.7.HiTrap® Desalting column allows easy and efficient group separations with a syringe, pump, or chromatography system.
HiTrap® Desalting is a 5 mL column (Figure 5.7) packed with the SEC medium Sephadex G-25 Superfine, which is based on cross-linked dextran beads. The fractionation range for globular proteins is between Mr 1000 and 5000, with an exclusion limit of approximately Mr 5000. This ensures group separations of proteins/peptides larger than Mr 5000 from molecules with a molecular weight less than Mr 1000.
HiTrap® Desalting can be used with aqueous solutions in the pH range 2 to 13. The prepacked medium is stable in all commonly used buffers, solutions of urea (8 M), guanidine hydrochloride (6 M), and all nonionic and ionic detergents. Lower alcohols (methanol, ethanol, propanol) can be used in the buffer or the sample, but we recommend that the concentration be kept below 25% v/v. Prolonged exposure (hours) to pH below 2 or above 13, or to oxidizing agents, should be avoided.
The recommended range of sample volumes is 0.1 to 1.5 mL when complete removal of low molecular weight components is desired. The separation is not affected by the flow rate, in the range of 1 to 10 mL/min. The maximum recommended flow rate is 15 mL/min. Separations are easily performed with a syringe, pump, or chromatography system. Up to three columns can be connected in series, allowing larger sample volumes to be handled. To avoid crosscontamination, use the column only with the same type of sample.
Manual purification with a syringe
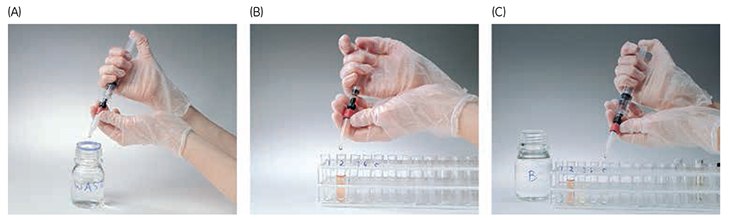
Figure 5.8.Using HiTrap® columns with a syringe. (A) Prepare buffers and sample. Remove the column’s top cap and twist off the end. (B) Equilibrate the column, load the sample, and begin collecting fractions. (C) Wash and elute, continuing to collect fractions.
- Fill the syringe with binding buffer. Remove the stopper and connect the column to the syringe (use the connector supplied) “drop to drop” to avoid introducing air into the column.
- Remove the snap-off end at the column outlet
- Equilibrate the column with 5 column volumes of binding buffer.
- Apply the pretreated sample using a syringe fitted to the Luer connector on the column. For optimal results, use a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) during sample application*.
- Wash with 5 to 10 column volumes of binding buffer or until no material appears in the effluent. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for washing. Optional: collect the flowthrough (in 1 mL fractions for the 1 mL column and 2 mL fractions for the 5 mL column) and reserve until the procedure has been successfully completed. Retain a sample for analysis by SDS-PAGE to measure the efficiency of protein binding to the medium.
- Elute with 5 to 10 column volumes of elution buffer. Maintain a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) for elution.
- After elution, regenerate the column by washing it with 3 to 5 column volumes of binding buffer. The column is now ready for a new purification.
* 1 mL/min corresponds to approximately 30 drops/min when using a syringe with a HiTrap® 1 mL column; 5 mL/min corresponds to approximately 120 drops/min when using a HiTrap® 5 mL column.
For large sample volumes, a peristaltic pump can be used to apply sample and buffers.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?