ALiCE®Cell-Free Protein Synthesis System Protocol
- Introduction to the ALiCE® Cell-Free Protein Expression System
- Contents of the ALiCE® Cell-Free Protein Expression Kit
- Additional Product Information
- ALiCE® Cell-Free Protein Expression Protocols
- ALiCE® Expression Vectors
- Template preparation by cloning into pALiCE vectors
- Alternatives
- Purification of DNA
- Coupled transcription/translation reaction setup
- Reaction assembly and reaction
- Recovery of proteins produced in the microsomes
- Detection of produced proteins
- Isolation of produced proteins
- Troubleshooting
- References
- Appendix A: pALiCE Plasmid Sequence Information
Introduction to the ALiCE® Cell-Free Protein Expression System
Cell-free protein synthesis (CFPS) or expression (CFPE) systems derived from crude cell extracts have been used for decades as a research tool and have introduced many attractive advantages to protein expression technology. However, a critical barrier to the adoption of CFPE systems as alternatives to cell-based approaches has been the problem of relatively low protein yields.
To address this problem, we have taken advantage of plant cell biology by developing a novel eukaryotic cell-free expression system that offers unprecedented yields of up to 3 mg/mL of protein in batch mode. The ALiCE® Cell-Free Protein Expression System enables you to overcome the limitations of living host systems and to obtain “difficult to produce” proteins in a matter of hours instead of weeks.
The ALiCE® system utilizes tobacco cell lysates, depleted of vacuoles. The system contains all factors required for in vitro transcription (RNA polymerases, NTPs) and translation reactions (ribosomes, translation initiation/elongation factors, tRNA, etc.). Using a simple protocol, both the RNA transcription and translation take place in a single tube (coupled transcription/translation).
Reactions are started by simply mixing plasmid DNA with the ALiCE® reaction mix. The outstanding protein yields of the ALiCE® system are based on highly efficient protein expression over the duration of the reaction. Consequently, we recommend a reaction duration of 48 hr.
The ALiCE® system comes with two expression vectors, pALiCE01 and pALiCE02, which are used according to the targeting of the protein of interest. These have been specially tailored to offer excellent protein yield when used with the ALiCE® expression system. pALiCE01 is used for expression in ALiCE® cytosolic fraction. Targeting the microsomes with pALiCE02 offers the opportunity to express protein with post-translational modifications like disulfide bonds.
Besides screening, the ALiCE® system can also be used for larger scale expression of target proteins. Potential additional applications of the ALiCE® system include, but are not limited to:
- Target protein characterization
- Protein optimization
- Mutant screening
- Protein-protein interactions
- Expression analysis
- Protein localization analysis
- Protein crystallization and structure analysis
- Post-translational modification assays
- Metabolomics
- Herbicide screening
Contents of the ALiCE® Cell-Free Protein Expression Kit
Equipment and material to be provided by user:
- Gloves
- Pipette filter tips (RNase-free)
- RNase-free water (e.g. water from a Millipore-filter unit)
- Orbital shaker
- When reactions performed in tubes:
Tabletop shaker (2 mL vessel insert), shaking diameter: 3 mm OR - When reactions performed in plates:
Shaker with controlled humidity (up to 70 %), shaking diameter: 25 mm with plate holder and 96 half well plates (RNase free)
- When reactions performed in tubes:
Additional Product Information
IMPORTANT NOTICES
- RNase contamination leads to lower or no protein yields. Only use RNase-free filter-tips and wear gloves at all times.
- The ALiCE® kit requires oxygen during the whole reaction time for a successful reaction. Do not seal the reaction vessels.
Shipping and Storage ALiCE® kits are shipped on dry ice. Upon arrival, immediately store the components as indicated in the table above. The reaction mix is stable for at least 12 months under these conditions.
Product Use Limitations The ALiCE® kit is for research purposes only. It is not to be used for diagnostic or preventive action or treatment of a disease, nor to be administered to humans. Please refer to the Limited Use Label License (LULL).
Product Warranty ALiCE® kits are shipped frozen on dry ice. If there is no dry ice remaining in the package upon delivery, or if the package is damaged, the quality of the kit may be compromised. Contact us immediately if any issues with the delivery have occurred. The warranty remains in effect up to the expiration date indicated on the product label.
Quality Assurance High quality chemicals and materials have been used to manufacture the components of ALiCE® kits. Each lot is carefully tested to ensure that all components meet the stated specifications (see Certificate of Analysis).
Safety The user should observe all applicable regulations for handling chemicals and recombinant DNA. Lab coat, safety glasses and gloves should be worn when handling the kit components. When handling ultra- cold material wear additional protective gloves to avoid frostbite. Used components are to be disposed of in accordance to the local regulations.
Disclaimer We assume no liability for any direct or indirect damages or loss arising from the use, misuse or results of use of the ALiCE® kit.
ALiCE® Cell-Free Protein Expression Protocols
Every use of the ALiCE® protein expression system follows a simple structure:
ALiCE® Expression Vectors
The ALiCE® kit is provided with a set of two vectors to serve different needs regarding protein expression:
1. pALiCE01 to express easy-to-produce proteins This plasmid will target your expressed protein to the cytosolic fraction in high yield applications. Posttranslational modifications are not possible with this expression vector.
2. pALiCE02 to express difficult-to-produce proteins with e.g. disulfide bonds etc. Microsomes are organelle-like structures that form from parts of the endoplasmic reticulum during the manufacturing process of the ALiCE® reaction mix. Using a melittin signal peptide, proteins can be translocated into these organelles (Buntru et al., 2015), where they undergo post-translational modification. Subsequently, the microsomes can be disrupted to recover the expressed proteins (see Page 10).
pALiCE01 and pALiCE02 can also both be used as a positive control for expression, expressing eYFP when added to the ALiCE® reaction mix in the cytosol and the microsomes, respectively. When using pALiCE01 as a positive control, a yellow color should be visible in the reaction mix after 48 hours.
Template Preparation by Cloning into pALiCE Vectors
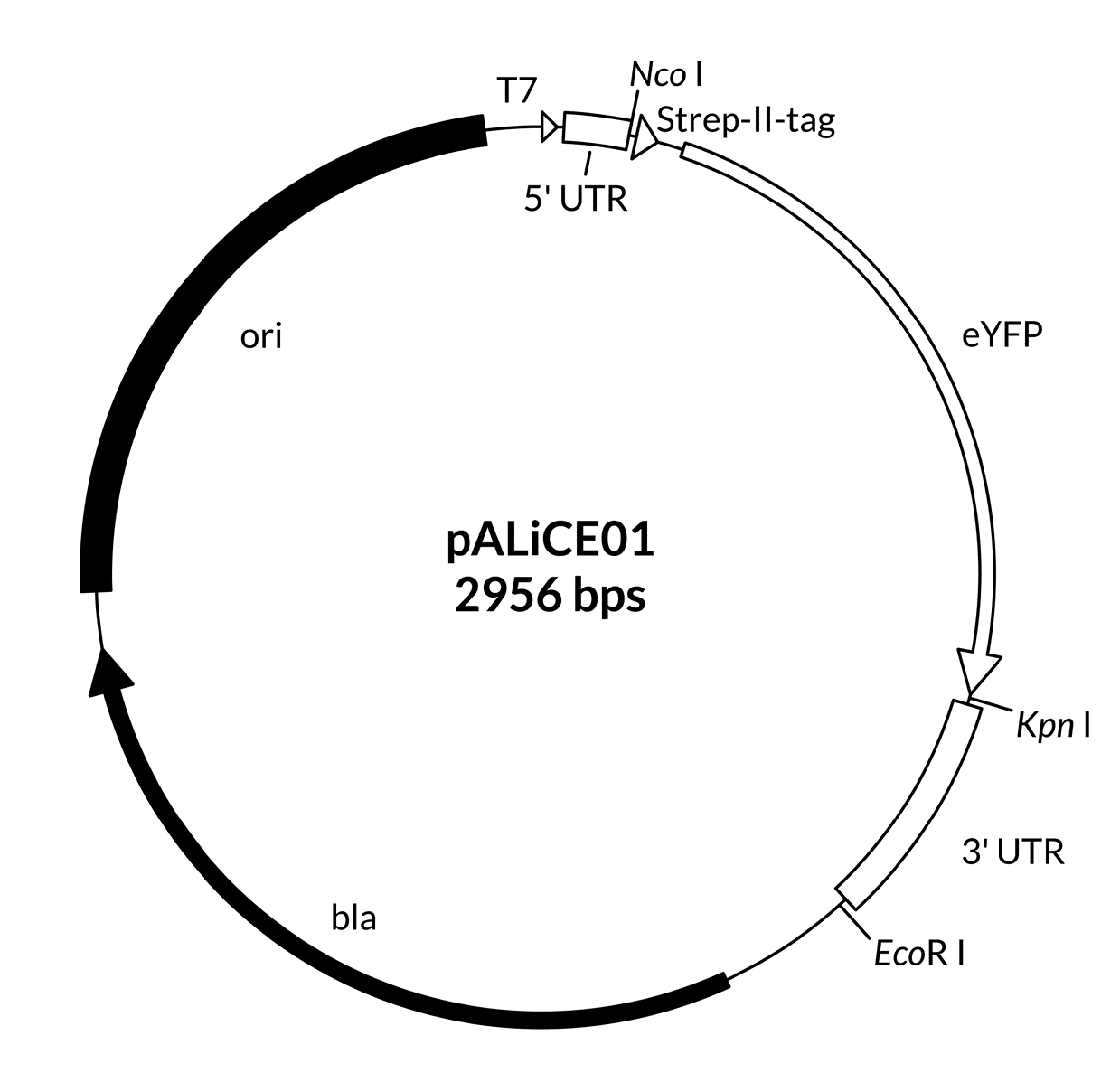
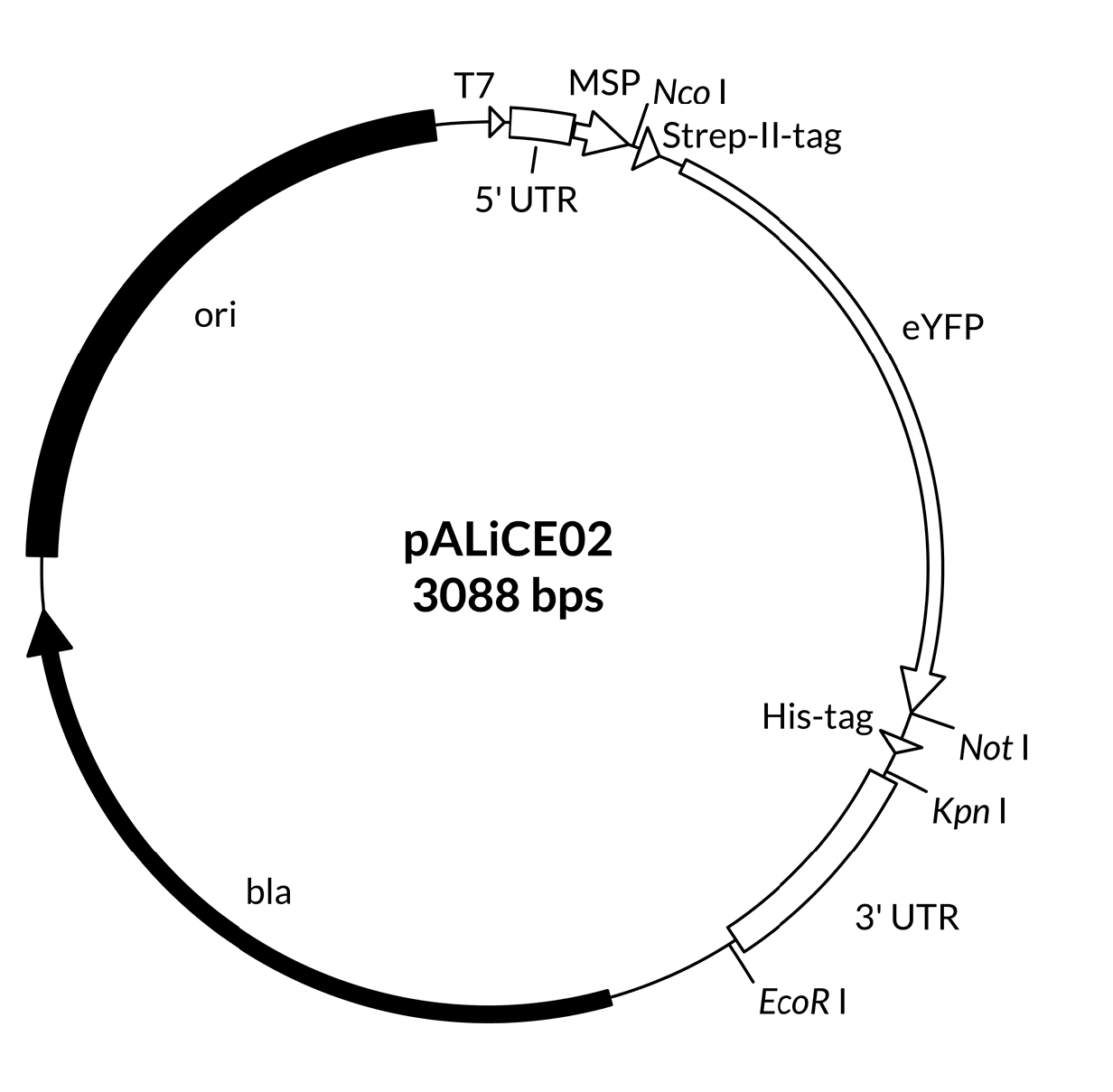
A map including the features of the pALiCE01 and pALiCE02 vectors is provided below (Figure 1), as well as expanded views of the cloning sides in 5’ and 3’ direction of the gene of interest (Figure 2 and Figure 3). Plasmid sequences are found in Appendix A.
The cloning site allows insertion of the gene of interest behind the T7 promoter and the 5' untranslated region. Cloning your gene of interest can either be done by subcloning from an existing vector or amplification of its coding region by PCR for the introduction of the appropriate restriction enzyme sites. Recommended restriction sites for the cytosolic expression plasmid pALiCE01 are Nco I and Kpn I while for the microsomal expression plasmid either Nco I / Not I or Nco I / Kpn I are recommended. If using Kpn I for cloning, please make sure the open reading frame contains a stop codon.
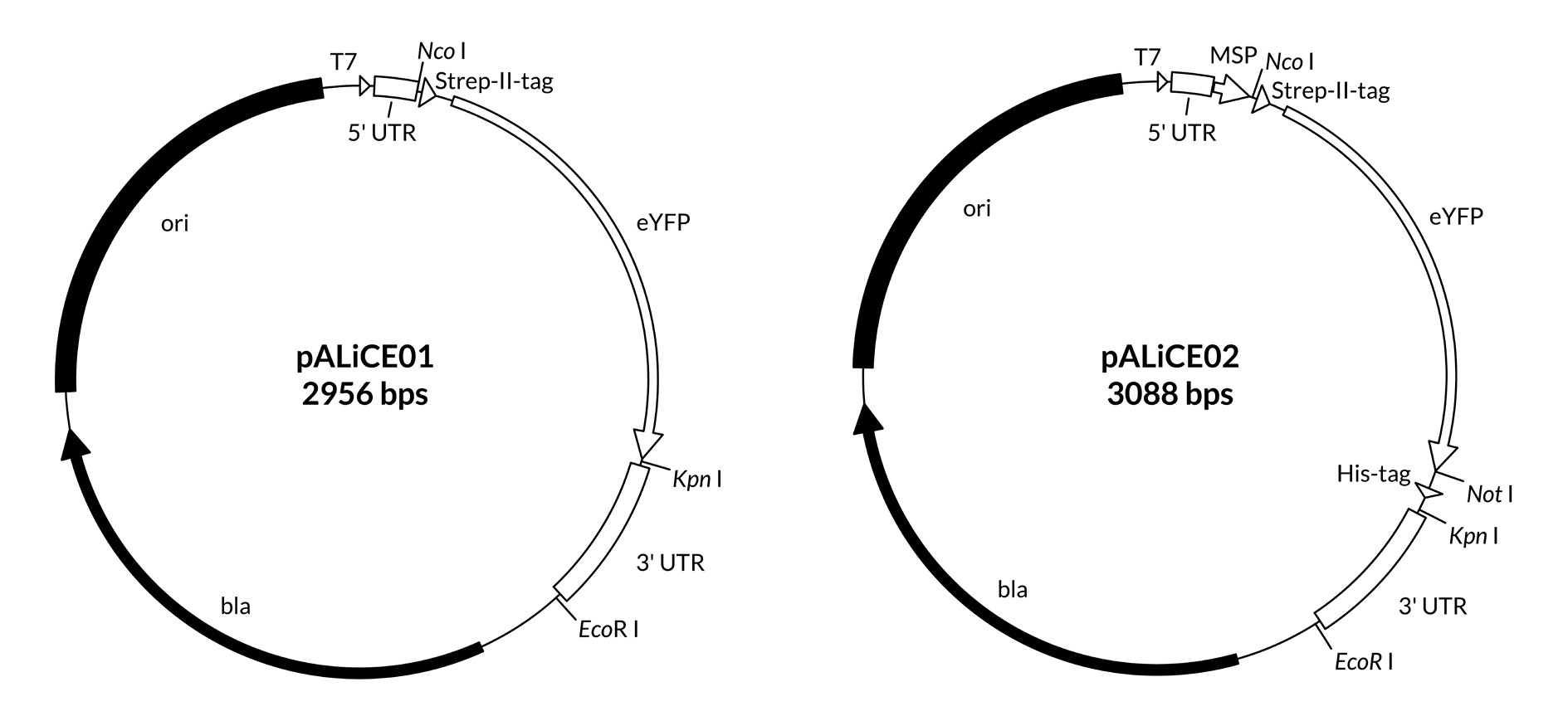
Figure 1: Map of pALiCE01 and pALiCE02 vectors and sequence reference points. Additional description: T7: T7 RNA polymerase promoter; 5’ UTR: 5’ untranslated region; MSP: melittin signal peptide; 3’UTR: 3’ untranslated region; bla: β-lactamase gene (resistant to ampicillin); ori: origin of plasmid replication.
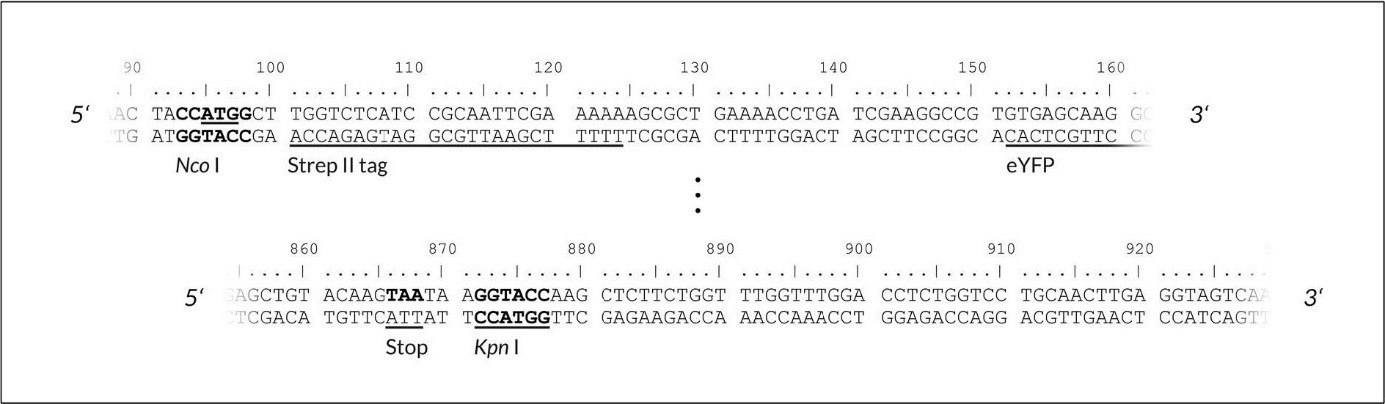
Figure 2:pALiCE01: View of the restriction sites of interest. Upper part: upstream cloning region of the gene of interest (here: eYFP). Lower part: downstream cloning region of the gene of interest. Please note, that the start Codon is a part of the restriction side of Nco I (underlined).
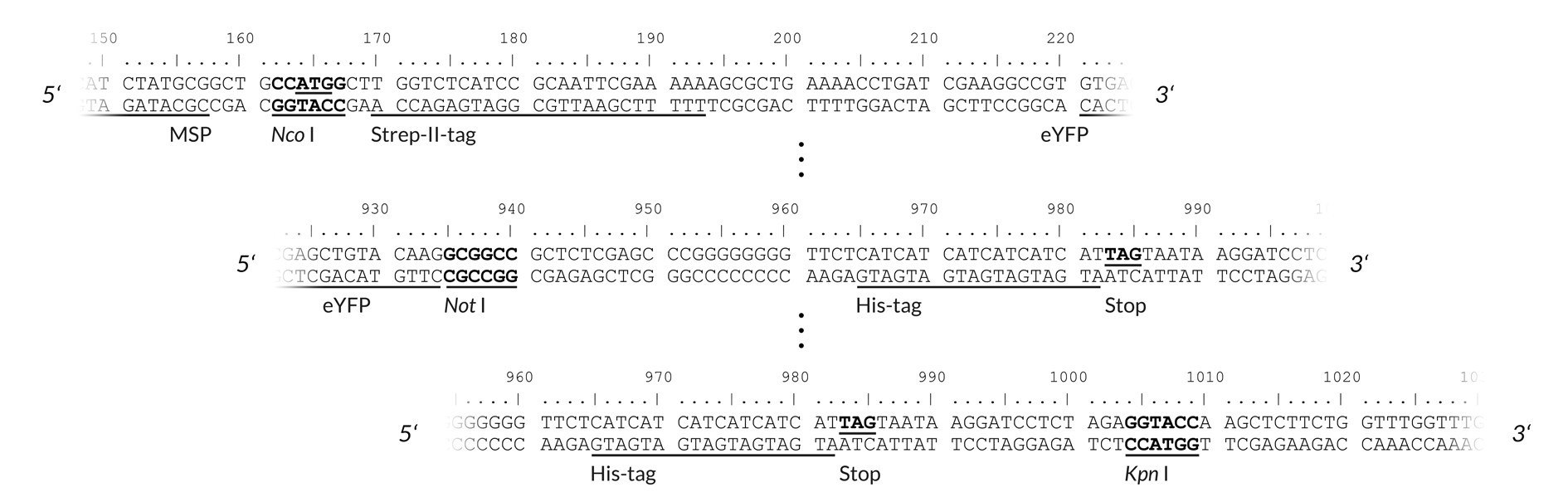
Figure 3:pALiCE02: View of the restriction sites of interest. Upper part: upstream cloning region of the gene of interest (here: eYFP). Lower part: downstream cloning region of the gene of interest. With choosing either Not I or Kpn I a His6-tag can be added. If using the Kpn I restriction site, please add a stop codon to your gene of interest.
After insertion of the gene of interest by standard cloning techniques (Green & Sambrook, 2012) the plasmid is transferred into DH5α competent cells. Use of other E. coli strains may have an adverse effect on target protein yield due to lower plasmid quality. The vector encodes a β-lactamase (bla) to allow selection on ampicillin containing media.
Note: Using other T7 based eukaryotic vectors may reduce protein yield and prokaryotic vectors are not applicable with the ALiCE® kit.
Alternative Methods
We recommend amplifying the plasmid with your target gene through standard cloning while utilizing the ALiCE® kit for your needs. However, to circumvent introducing the plasmid into any cells to save time and labor, various methods are described for different cell-free systems, including the Rolling Circle method (Wang et al., 2016, and Kumar & Chernaya, 2009). Synthesized plasmids containing your gene of interest by a 3rd party and applying directly to the lysate has not been validated for high-yield production with the ALiCE® kit.
Purification of DNA
To facilitate an efficient transcription-translation reaction, a highly purified plasmid DNA preparation is required. It is therefore highly recommended to use a plasmid preparation procedure based on anion exchange chromatography (e.g. NucleoBond® Xtra Midi from Macherey-Nagel). For plasmid DNA preparation kits based on silica matrices we recommend addition of RNase inhibitor or phenol- chloroform purification of the template prior to the transcription-translation reaction.
Adjust the final plasmid DNA to the desired concentration depending on the size of the insert by adding an appropriate volume of 5 mM Tris buffer (pH 8.5). The final concentration in the reaction mix should be 5 nM. Store in aliquots at -20°C.
Coupled transcription/translation reaction setup
The typical reaction volume for coupled transcription-translation reaction is 50 µL.
1. Choose the reaction vessel
•ALiCE® tubes = 52 µL per reaction
•96 half well plates with lid1 = 50 µL per reaction
2. Prepare DNA Remove plasmid DNA template from storage and thaw at room temperature (20 - 25 °C). Mix gently by flipping and briefly spin down to collect the content at the bottom of the tube then place on ice. Dilute or concentrate plasmid, the final concentration in the assembled reaction should be 5 nM. DNA concentration may significantly affect protein yield.
3. Thaw ALiCE® reaction mix1 Remove plasmid DNA template from storage and thaw at room temperature (20 - 25 °C). Mix gently by flipping and briefly spin down to collect the content at the bottom of the tube then place on ice. Dilute or concentrate plasmid, the final concentration in the assembled reaction should be 5 nM. DNA concentration may significantly affect protein yield.
1 For best results, use greiner bio-one, Art. No. 675086
Reaction assembly and reaction cycle
ALiCE®Tubes
ALiCE® Tubes Assemble in ALiCE® tube according to the following table:
- Only use the supplied punctured caps to close ALiCE® tubes.
- Incubate the ALiCE® tubes in an orbital tabletop shaker at 700 rpm and 25 °C for 48 hr. We recommend a shaking diameter of 3 mm and the use of a holding block capable of holding 2 mL vessels for optimal results.
Note: Constant oxygen supply is needed throughout the complete reaction cycle. Therefore, the reaction volume in the tubes can be increased up to 200 µL however, there may be decreased reaction productivity. For reaction volumes above 200 µL it is recommended to divide the sample into smaller volumes.
96 half well plates
Assemble in 96 half well plate according to the following table :
- Pipette water into the spaces between the reaction compartments of the plate (75 µL each) to maintain a high humidity inside the plate during incubation.
- Incubate the plates in an orbital shaker at 500 rpm and 25 °C for 48 hr. We recommend a 12.5 mm or 25 mm shaking diameter and controlled humidity of > 70 % for optimal results. If no humidity control is available, it is recommended to put open containers filled with water in the shaker to provide a higher than ambient relative humidity.
Note: Protein expression is still possible at humidity levels below 70%, although evaporation may occur and protein expression yield may be diminished. Do not seal reaction vessels or plates.
Note on DNA template concentration
Calculation of the concentration of the DNA template to use (5 nM) may be circumvented by approximating the necessary mass of the template according to following formula
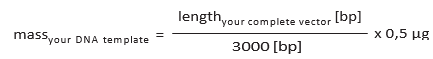
Recovery of proteins produced in the microsomes
Expressing proteins with the ALiCE® kit using the pALiCE02 vector will result in the target protein being produced in the microsomes of the reaction mix. To release the target protein from the microsomes, the following steps are taken:
- Add 0.5% n-dodecyl-β-maltoside (DDM) to the ALiCE® reaction in order to lyse microsomes harboring the synthesized protein.
- Incubate for 10 min at room temperature with shaking at 700 rpm. Do not vortex.
- Centrifuge the lysis reaction at 16,000 x g for 10 min at room temperature. Transfer the supernatant containing the synthesized protein to a new reaction tube.
- Keep in mind that the DDM in the supernatant may affect subsequent steps taken, e.g. activity assays or chromatography purification.
Detection of synthesized proteins
Synthesized target proteins can be detected by SDS-PAGE, which requires high resolution and an appropriate gel concentration in order to ensure a good separation of the expressed protein from background proteins. We recommend an SDS-PAGE gradient gel and loading of a maximum of 1 µL of sample. Also, we recommend a 10 min heat treatment of the sample at 99 °C when performing a denaturing SDS- PAGE.
Isolation of produced proteins
Recombinant proteins produced with the ALiCE® system can be purified with conventional chromatography separation methods, e.g. affinity tag chromatography.
Sequence
TAATACGACTCACTATAGGGAGAGTATTTTTACAACAATTACCAACAACAACAACAAACAACAACAAC ATTACATTTTACATTCTACAACTACCATGGCTTGGTCTCATCCGCAATTCGAAAAAAGCGCTGAAAAC CTGATCGAAGGCCGTGTGAGCAAGGGCGAGGAGCTGTTCACCGGGGTGGTGCCCATCCTGGTCGAG CTGGACGGCGACGTAAACGGCCACAAGTTCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTAC GGCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCTCGTGA CCACCTTCGGCTACGGCCTGCAGTGCTTCGCCCGCTACCCCGACCACATGAAGCAGCACGACTTCTT CAAGTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTAC AAGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGGGCATC GACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTACAACTACAACAGCCACAACGTCT ATATCATGGCCGACAAGCAGAAGAACGGCATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGA CGGCAGCGTGCAGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTGCTGCT GCCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGACCCCAACGAGAAGCGCGATCAC ATGGTCCTGCTGGAGTTCGTGACCGCCGCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGTAAT AAGGTACCAAGCTCTTCTGGTTTGGTTTGGACCTCTGGTCCTGCAACTTGAGGTAGTCAAGATGCATA ATAAATAACGGATTGTGTCCGTAATCACACGTGGTGCGTACGATAACGCATAGTGTTTTTCCCTCCAC TTAAATCGAAGGGTTGTGTCTTGGATCGCGCGGGTCAAATGTATATGGTTCATATACATCCGCAGGCA CGTAATAAAGCGAGGGGTTCGAATCCCCCCGTTACCCCCGGTAGGGGCCCATTATAGCCGAATTCGG CGCGCCAGGTGGCACTTTTCGGGGAAATGTGCGCGGAACCCCTATTTGTTTATTTTTCTAAATACATT CAAATATGTATCCGCTCATGAGACAATAACCCTGATAAATGCTTCAATAATATTGAAAAAGGAAGAGT ATGAGTATTCAACATTTCCGTGTCGCCCTTATTCCCTTTTTTGCGGCATTTTGCCTTCCTGTTTTTGCTC ACCCAGAAACGCTGGTGAAAGTAAAAGATGCTGAAGATCAGTTGGGTGCACGAGTGGGTTACATCGA ACTGGATCTCAACAGCGGTAAGATCCTTGAGAGTTTTCGCCCCGAAGAACGTTTTCCAATGATGAGCA CTTTTAAAGTTCTGCTATGTGGCGCGGTATTATCCCGTATTGACGCCGGGCAAGAGCAACTCGGTCGC CGCATACACTATTCTCAGAATGACTTGGTTGAGTACTCACCAGTCACAGAAAAGCATCTTACGGATGG CATGACAGTAAGAGAATTATGCAGTGCTGCCATAACCATGAGTGATAACACTGCGGCCAACTTACTTC TGACAACGATCGGAGGACCGAAGGAGCTAACCGCTTTTTTGCACAACATGGGGGATCATGTAACTCG CCTTGATCGTTGGGAACCGGAGCTGAATGAAGCCATACCAAACGACGAGCGTGACACCACGATGCCT GTAGCAATGGCAACAACGTTGCGCAAACTATTAACTGGCGAACTACTTACTCTAGCTTCCCGGCAACA ATTAATAGACTGGATGGAGGCGGATAAAGTTGCAGGACCACTTCTGCGCTCGGCCCTTCCGGCTGGC TGGTTTATTGCTGATAAATCTGGAGCCGGTGAGCGTGGTTCTCGCGGTATCATTGCAGCACTGGGGC CAGATGGTAAGCCCTCCCGTATCGTAGTTATCTACACGACGGGGAGTCAGGCAACTATGGATGAACG AAATAGACAGATCGCTGAGATAGGTGCCTCACTGATTAAGCATTGGTAACTGTCAGACCAAGTTTACT CATATATACTTTAGATTGATTTAAAACTTCATTTTTAATTTAAAAGGATCTAGGTGAAGATCCTTTTTGA TAATCTCATGACCAAAATCCCTTAACGTGAGTTTTCGTTCCACTGAGCGTCAGACCCCGTAGAAAAGA TCAAAGGATCTTCTTGAGATCCTTTTTTTCTGCGCGTAATCTGCTGCTTGCAAACAAAAAAACCACCGC TACCAGCGGTGGTTTGTTTGCCGGATCAAGAGCTACCAACTCTTTTTCCGAAGGTAACTGGCTTCAGC AGAGCGCAGATACCAAATACTGTTCTTCTAGTGTAGCCGTAGTTAGGCCACCACTTCAAGAACTCTGT AGCACCGCCTACATACCTCGCTCTGCTAATCCTGTTACCAGTGGCTGCTGCCAGTGGCGATAAGTCGT GTCTTACCGGGTTGGACTCAAGACGATAGTTACCGGATAAGGCGCAGCGGTCGGGCTGAACGGGGG GTTCGTGCACACAGCCCAGCTTGGAGCGAACGACCTACACCGAACTGAGATACCTACAGCGTGAGCT ATGAGAAAGCGCCACGCTTCCCGAAGGGAGAAAGGCGGACAGGTATCCGGTAAGCGGCAGGGTCGG AACAGGAGAGCGCACGAGGGAGCTTCCAGGGGGAAACGCCTGGTATCTTTATAGTCCTGTCGGGTTT CGCCACCTCTGACTTGAGCGTCGATTTTTGTGATGCTCGTCAGGGGGGCGGAGCCTATGGAAAAACG CCAGCAACGCGGCCTTTTTACGGTTCCTGGCCTTTTGCTGGCCTTTTGCTCACATG
pALiCE02:
Sequence
TAATACGACTCACTATAGGGAGAGTATTTTTACAACAATTACCAACAACAACAACAAACAACAACAAC ATTACATTTTACATTCTACAACTACCATGAAATTCTTAGTCAACGTTGCCCTTGTTTTTATGGTCGTATA CATTTCTTACATCTATGCGGCTGCCATGGCTTGGTCTCATCCGCAATTCGAAAAAAGCGCTGAAAACC TGATCGAAGGCCGTGTGAGCAAGGGCGAGGAGCTGTTCACCGGGGTGGTGCCCATCCTGGTCGAGC TGGACGGCGACGTAAACGGCCACAAGTTCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACG GCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCTCGTGAC CACCTTCGGCTACGGCCTGCAGTGCTTCGCCCGCTACCCCGACCACATGAAGCAGCACGACTTCTTC AAGTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACA AGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGGGCATCG ACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTACAACTACAACAGCCACAACGTCTA TATCATGGCCGACAAGCAGAAGAACGGCATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGAC GGCAGCGTGCAGCTCGCCGACCACTACCAGCAGAACACCCCCATCGGCGACGGCCCCGTGCTGCTG CCCGACAACCACTACCTGAGCTACCAGTCCGCCCTGAGCAAAGACCCCAACGAGAAGCGCGATCACA TGGTCCTGCTGGAGTTCGTGACCGCCGCCGGGATCACTCTCGGCATGGACGAGCTGTACAAGGCGGC CGCTCTCGAGCCCGGGGGGGGTTCTCATCATCATCATCATCATTAGTAATAAGGATCCTCTAGAGGTA CCAAGCTCTTCTGGTTTGGTTTGGACCTCTGGTCCTGCAACTTGAGGTAGTCAAGATGCATAATAAAT AACGGATTGTGTCCGTAATCACACGTGGTGCGTACGATAACGCATAGTGTTTTTCCCTCCACTTAAAT CGAAGGGTTGTGTCTTGGATCGCGCGGGTCAAATGTATATGGTTCATATACATCCGCAGGCACGTAAT AAAGCGAGGGGTTCGAATCCCCCCGTTACCCCCGGTAGGGGCCCATTATAGCCGAATTCGGCGCGCC AGGTGGCACTTTTCGGGGAAATGTGCGCGGAACCCCTATTTGTTTATTTTTCTAAATACATTCAAATAT GTATCCGCTCATGAGACAATAACCCTGATAAATGCTTCAATAATATTGAAAAAGGAAGAGTATGAGTA TTCAACATTTCCGTGTCGCCCTTATTCCCTTTTTTGCGGCATTTTGCCTTCCTGTTTTTGCTCACCCAGA AACGCTGGTGAAAGTAAAAGATGCTGAAGATCAGTTGGGTGCACGAGTGGGTTACATCGAACTGGAT CTCAACAGCGGTAAGATCCTTGAGAGTTTTCGCCCCGAAGAACGTTTTCCAATGATGAGCACTTTTAA AGTTCTGCTATGTGGCGCGGTATTATCCCGTATTGACGCCGGGCAAGAGCAACTCGGTCGCCGCATA CACTATTCTCAGAATGACTTGGTTGAGTACTCACCAGTCACAGAAAAGCATCTTACGGATGGCATGAC AGTAAGAGAATTATGCAGTGCTGCCATAACCATGAGTGATAACACTGCGGCCAACTTACTTCTGACAA CGATCGGAGGACCGAAGGAGCTAACCGCTTTTTTGCACAACATGGGGGATCATGTAACTCGCCTTGA TCGTTGGGAACCGGAGCTGAATGAAGCCATACCAAACGACGAGCGTGACACCACGATGCCTGTAGCA ATGGCAACAACGTTGCGCAAACTATTAACTGGCGAACTACTTACTCTAGCTTCCCGGCAACAATTAAT AGACTGGATGGAGGCGGATAAAGTTGCAGGACCACTTCTGCGCTCGGCCCTTCCGGCTGGCTGGTTT ATTGCTGATAAATCTGGAGCCGGTGAGCGTGGTTCTCGCGGTATCATTGCAGCACTGGGGCCAGATG GTAAGCCCTCCCGTATCGTAGTTATCTACACGACGGGGAGTCAGGCAACTATGGATGAACGAAATAG ACAGATCGCTGAGATAGGTGCCTCACTGATTAAGCATTGGTAACTGTCAGACCAAGTTTACTCATATA TACTTTAGATTGATTTAAAACTTCATTTTTAATTTAAAAGGATCTAGGTGAAGATCCTTTTTGATAATCT CATGACCAAAATCCCTTAACGTGAGTTTTCGTTCCACTGAGCGTCAGACCCCGTAGAAAAGATCAAAG GATCTTCTTGAGATCCTTTTTTTCTGCGCGTAATCTGCTGCTTGCAAACAAAAAAACCACCGCTACCAG CGGTGGTTTGTTTGCCGGATCAAGAGCTACCAACTCTTTTTCCGAAGGTAACTGGCTTCAGCAGAGCG CAGATACCAAATACTGTTCTTCTAGTGTAGCCGTAGTTAGGCCACCACTTCAAGAACTCTGTAGCACC GCCTACATACCTCGCTCTGCTAATCCTGTTACCAGTGGCTGCTGCCAGTGGCGATAAGTCGTGTCTTA CCGGGTTGGACTCAAGACGATAGTTACCGGATAAGGCGCAGCGGTCGGGCTGAACGGGGGGTTCGT GCACACAGCCCAGCTTGGAGCGAACGACCTACACCGAACTGAGATACCTACAGCGTGAGCTATGAGA AAGCGCCACGCTTCCCGAAGGGAGAAAGGCGGACAGGTATCCGGTAAGCGGCAGGGTCGGAACAG GAGAGCGCACGAGGGAGCTTCCAGGGGGAAACGCCTGGTATCTTTATAGTCCTGTCGGGTTTCGCCA CCTCTGACTTGAGCGTCGATTTTTGTGATGCTCGTCAGGGGGGCGGAGCCTATGGAAAAACGCCAGC AACGCGGCCTTTTTACGGTTCCTGGCCTTTTGCTGGCCTTTTGCTCACATG
ALiCE is a registered trademark of LenioBio GmbH in Germany. NucleoBond is a registered trademark of Macherey-Nagel. Limited Use Label License for Research Only
References
To continue reading please sign in or create an account.
Don't Have An Account?