Large Volume Concentration and Diafiltration with Amicon® Stirred Cells
Commonly, sample concentration is performed first to reduce the overall sample volume, followed by diafiltration. This approach significantly reduces the amount of diafiltration buffer required. However, if a sample is unstable or too viscous at higher concentration, a partial concentration may be performed first, followed by diafiltration. The final concentration step is then performed in the exchange buffer. This method will use more exchange buffer, but will maintain a greater permeate flux due to lower concentration or viscosity, reducing the process time and ultimately protecting sample integrity.
To demonstrate the utility of the Amicon® Stirred Cell for large volume concentration, a 10x concentration was performed, reducing 500 mL of a 0.1 mg/mL BSA solution with 1 M NaCl to a final volume of 50 mL.
The experiment was performed using:
- 200 mL Amicon® Stirred Cell (cat. no. UFSC20001)
- 800 mL Amicon® Stirred Cell Reservoir (cat. no. 6028) to expand the total diafiltration volume range
- Protein concentration and diafiltration were performed using a 10 kDa Regenerated Cellulose membrane (cat. no. PLGC06210)
- To enable quick and simple switching between concentration and diafiltration modes without interrupting system operation, the Amicon® Stirred Cell Selector Valve (cat. no. 6003) was installed between the external reservoir and the stirred cell
Continuous Diafiltration Setup
Figure 1.Continuous diafiltration setup using the Amicon® Stirred Cell Selector Valve and Amicon Stirred Cell Reservoir accessories.
Method 1. Large Volume Concentration
Setup
- Following the user guide instructions for the selector valve, the inlet/outlet tube fittings were attached to the appropriate tubing.
- Both the Amicon® Stirred Cell and reservoir were assembled, and the reservoir was placed into the retaining stand.
For 10x concentration of 500 mL of 0.1 mg/mL BSA in 1 M NaCl
- 200 mL was added to the stirred cell and the remainder was added to the reservoir through the recessed sample port.
- The Amicon stirred cell was placed onto a magnetic stirrer.
NOTE: To reduce hold-up volume, care should be taken to minimize tubing length. If necessary, the reservoir should be tilted toward the inlet tubing to assure that all the sample or buffer is transferred to the Amicon stirred cell during processing. - The selector valve was set to “Gas” mode (gas spool in)
- Stirring was initiated at 200 rpm.
- Nitrogen gas was applied at 50 psi, pressurizing the Amicon® Stirred Cell and the reservoir.
- The pressure was thus equalized over the liquid volume in both the Amicon stirred cell and the reservoir, allowing the sample to concentrate.
- Once the BSA solution in the Amicon® Stirred Cell was concentrated to approximately 50 mL, the selector valve was switched to “Liquid” mode (liquid spool in). This allowed pressurized liquid to flow out of the reservoir and into the Amicon stirred cell.
- The liquid level in the Amicon stirred cell was maintained at about 50 mL during the process.
- The filtration was stopped when the filtrate reached 450 mL and the concentrate was at 50 mL (10x concentration).
- The pressure and magnetic stirring were turned off and pressure was vented from both devices.
- BSA concentrations in the retentate and starting material were measured using A280nm to assure that the final concentration of 1 mg/mL BSA was reached.
Method 2. Continuous Diafiltration
The concentrated sample (now at 1 mg/mL BSA containing 1 M NaCl) was buffer-exchanged to remove the sodium chloride, using the previously described stirred cell accessories.
Setup
- The reservoir was disassembled using the cap removal tool (included in the reservoir kit), cleaned with mild detergent and rinsed with deionized water prior to refilling with 10 mM Tris HCl for salt removal.
- All fluid-carrying tubing was washed with mild detergent and rinsed with deionized water.
- The reservoir was again connected to the stirred cell containing the concentrated BSA (1 mg/mL with 1M NaCl) via the selector valve.
- The conductivity of the starting material was measured prior to desalting to monitor the progress of diafiltration.
Desalting
- The selector valve was set to “Gas” mode (gas spool in) and stirring was initiated on the magnetic stirrer at 200 rpm.
- Pressure was applied at 50 psi, pressurizing both the Amicon® Stirred Cell and the reservoir.
- After 5-10 seconds, when the pressure was equalized in both Amicon stirred cell and the reservoir, the selector valve was shifted to “Liquid” mode (liquid spool in), allowing the liquid in the reservoir to flow into the Amicon stirred cell.
- The liquid level in the Amicon stirred cell was maintained at about 50 mL during the desalting process.
- The protein concentration and salt conductivity of the concentrate was measured throughout the process to calculate salt reduction over time.
- The filtration process was stopped once the salt was reduced by 99%.
Method 3. Discontinuous Diafiltration
As previously described, 0.1 mg/mL BSA solution containing 1M NaCl was concentrated to 1 mg/mL BSA using large volume concentration. The concentrated sample (now at 1 mg/mL BSA containing 1 M NaCl), was then buffer-exchanged to remove the sodium chloride by discontinuous diafiltration using the Amicon® Stirred Cell.
Setup
- Amicon® Stirred Cell was disconnected from the selector valve.
- Stirred cell pressure inlet tubing was directly connected to a pressure-regulated nitrogen source.
Discontinuous Diafiltration
- 50 mL of 10 mM Tris HCl was added to the 50 mL of concentrated BSA solution in the Amicon stirred cell.
- The cap was reinstalled, the slide lock engaged and stirring was initiated prior to application of 50 psi pressure.
- Concentration continued until 50 mL of permeate was collected.
- Pressure was turned off at the source prior to removal of the cap.
- Protein concentration as well as conductivity measurements were performed on the retentate.
- To continue, the retentate was once again diluted with 50 mL of 10 mM Tris HCl and the process of concentration and dilution was continued until the salt concentration was reduced by 99%.
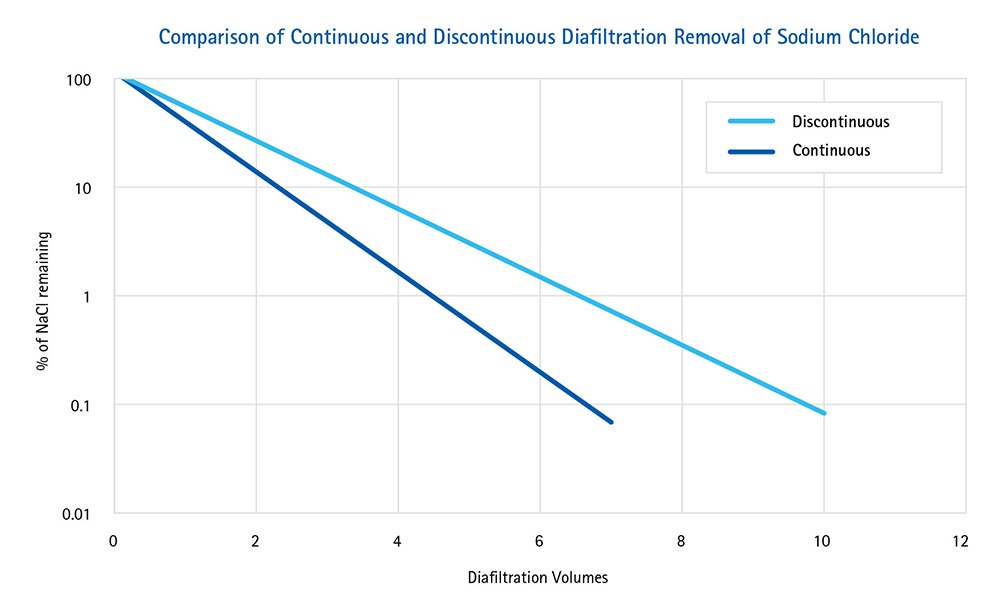
Figure 1.
Fewer DVs are required to remove sodium chloride using continuous diafiltration compared to discontinuous diafiltration. Data from the preceding table were plotted to show that continuous diafiltration is more efficient than the discontinuous method.
Discontinuous and Continuous Diafiltration Conclusion
Our results show that continuous diafiltration using the Amicon® Stirred Cells enables more efficient buffer exchange with less diafiltration volumes to reach 99% salt reduction compared to discontinuous diafiltration. Furthermore, in continuous mode, the stirred cell does not have to be disassembled between each diafiltration volume.
During continuous diafiltration, the protein concentration stayed constant at 1 mg/mL throughout the process, while in discontinuous diafiltration, the protein concentration fluctuated significantly between 1 mg/mL and 0.5 mg/mL. Therefore, continuous diafiltration is much more gentle than discontinuous diafiltration, as it maintains product stability by keeping the sample concentration and volume constant during diafiltration.
The flexible, easy-to-use Amicon® Stirred Cells are compatible with a broad range of process volumes (up to 400 mL) that can be further expanded with an addition of an external reservoir, which can be used for large volume concentration as well as batch and constant-volume diafiltration.
The new design of the Amicon® Stirred Cells accommodates a wide range of ultrafiltration and microfiltration disc membranes, which can be used to optimize concentration and diafiltration conditions. Unlike centrifugal devices, the pressure-based format provides a gentler method for concentration, reducing the likelihood of shear stress-induced denaturation.
Further, the inclusion of magnetic stirring at the filtration interface greatly minimizes the risk of concentration polarization and fouling.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?