Protocol for Thiol-Modified Oligonucleotide DDT Reduction
Reducing Disulfide Bonds
This protocol is for reducing the disulfide bond of thiol-modified oligonucleotides to the active sulfhydryl form (Figure 1). Sulfhydryl groups can be used for attaching oligonucleotides to solid surfaces, such as gold nanoparticles. Reduction of the disulfide bonds using Dithiothreitol (DTT), also known as Cleland's reagent, also protects against primer dimer.
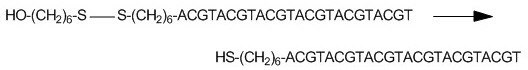
Figure 1. Example disulfide bond reduction of a 5'-Thiol-Modifier C6 S-S oligonucleotide. Thiol-modified oligonucleotides are shipped in disulfide form to prevent spontaneous, uncontrolled oxidation, which in turn would lead to dimer formation, thereby rendering the oligonucleotide useless.
Supplies
- 2 mL centrifuge tubes
- Pipette tips
- Milli-Q® H2O
- DTT (D9779)
- NAP™-10 column (GE17-0854-01)
- Sodium phosphate buffer
- Thiol-modified oligonucleotide
Method
The method is divided into two main steps: 1) sulfhydryl formation, and 2) byproduct removal.
Sulfhydryl Formation
- Prepare a 100 mM solution of DTT in 100 mM sodium phosphate buffer, pH 8.3 - 8.5. For 5 mL, dissolve 77.13 mg of DTT in buffer.
- For up to 12.5 OD (optical density units; for >12.5 OD, maintain a 10:1 ratio of DTT solution to OD of oligonucleotide), dissolve the thiol-modified oligonucleotide in 125 µL of the DTT solution.
- Incubate at room temperature for 1 hr.
Byproduct Removal
- Equilibrate a NAP-10 column with approximately 15 mL of 100 mM sodium phosphate buffer, pH 6.0. Allow the equilibration buffer to enter the gel bed completely.
- Add 1.0 mL of the reaction mixture to the NAP-10 column (bring the reaction mixture volume to 1.0 mL with sodium phosphate buffer, pH 6.0 as needed). Allow the reaction mixture to enter the gel bed completely.
- Elute the thiol-modified oligonucleotide into a centrifuge tube with 1.0 mL of sodium phosphate buffer, pH 6.0.
Additional Notes
Prepare sodium phosphate buffer, both at pH 8.3 - 8.5 and 6.0 according to standard laboratory practices. Make all buffers with Milli-Q® water.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?