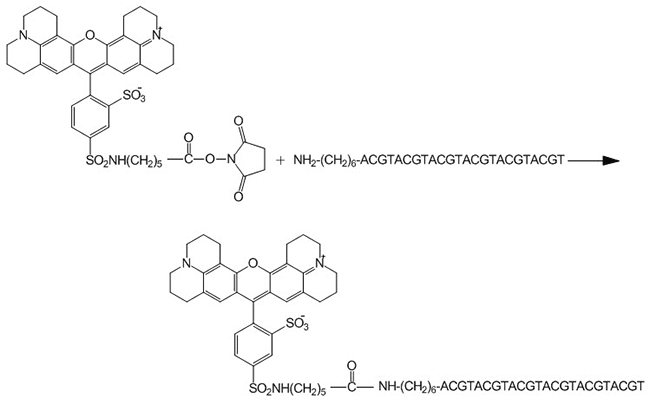
Protocol for Conjugating NHS-Ester Modifications to Amino-Labeled Oligonucleotides
This protocol is for conjugating NHS-ester modifications, such as TxRd (Sulforhodamine 101-X) (protocol is effective for most NHS-ester modifications), to an oligonucleotide with an amino label, such as 5'-Amino-Modifier C6 (Figure 1). Since TxRd (Sulforhodamine 101-X) and several other modifications are only available as NHS esters, they must be manually conjugated to amino-labeled oligonucleotides in a separate, post-synthesis reaction.
Definitions / Abbreviations
- DMSO - Dimethyl Sulfoxide
- EtOH - Ethanol
- HCl - Hydrochloric acid
- NaCl - Sodium chloride
- NaB - Sodium tetraborate decahydrate (Na2B4O7 ּ· 10H2O)
Equipment
- Laboratory shaker
- Refrigerated centrifuge (Eppendorf™ 5810R or equivalent)
- Lyophilizer or centrifugal evaporator (for non-air drying)
Supplies
- 1 mL syringe
- 20-gauge needle
- 2 mL centrifuge tubes
- 50 mL centrifuge tubes (optional)
- Pipette tips
- Disposable pipettes
- NaB (Product No. S9640)
- Milli-Q® H2O
- HCl (concentrated)
- 100% EtOH (Product No. E7023-4L) at –70 °C
- 70% EtOH at –20 °C
- 3M Sodium Acetate Buffer (Product No. S7899-1L)
- NHS-ester modification of choice
- 5'-Amino-Modifier C6 oligonucleotide with custom sequence
Method
The conjugation process is divided into two main steps: 1) the conjugation reaction, and 2) the removal of excess, free NHS-ester modification by post-conjugation precipitation.
Though HPLC is the conventional method of removing excess, free NHS-ester modification and uncoupled oligonucleotide, other purification processes, e.g. precipitation, may be effective too. Precipitation (described below) removes most but not all remaining NHS-ester modification. Depending on the application, the unconjugated modification may be a problem. While it will not react further since the NHS-ester is hydrolyzed by the buffer, it may contribute to background noise and therefore lead to a poor S:N ratio (if the modification is a dye, e.g. TxRd (Sulforhodamine 101-X) or reduce surface-loading density (if the modification serves to attach, e.g. biotin). In addition, uncoupled oligonucleotide will remain.
Conjugation Reaction
Start with the recommended conditions and optimize as needed.
- Ensure all reagents are thawed, thoroughly mixed, and centrifuged prior to use.
- Dissolve the amino-labeled oligonucleotide in a conjugation buffer, such as NaB pH 8.5, according to the guidelines in Table 1. The final oligonucleotide concentration will be between 0.3 and 0.8 mM. See Additional Notes for the Preparation of 50 mL of 0.091 M NaB Buffer.
- Dissolve the NHS-ester modification in DMSO. The NHS-ester modification concentration is approximately 14 mM (may deviate due to the variable molecular weight of the modification). See Additional Notes for the Preparation of NHS-Ester Modifications.
- Add the recommended volume of the NHS-ester modification to the recommended volume of the amino-labeled oligonucleotide according to the guidelines in Table 1. Gently vortex the tube. The indicated volumes will fit in a 2 mL tube. For larger-scale reactions, use either multiple 2 mL tubes or a 50 mL tube.
- Shake the tube(s) for 2 hours at room temperature (approximately 25 °C). Set the shaker speed appropriately to avoid splashing up the tube wall. To protect dyes from photobleaching, cover the tube(s) with aluminum foil.
Post-Conjugation Precipitation
The post-conjugation precipitation is performed in a 2 mL tube (50 mL tube for a larger reaction scale). Be sure to protect dyes from photobleaching.
- Add the volume of EtOH / 3M sodium acetate (9:1 V/V) solution to the conjugation reaction volume according to the guidelines in Table 1. Vortex the solution to initiate the precipitation. The EtOH / 3M sodium acetate solution should be prepared fresh (good for one week) using absolute EtOH.
- Spin the tube for 20 min at 4 °C. Use 3,000 RPM for an Eppendorf 5810R-style centrifuge or 13,000 RPM for a microcentrifuge.
- Use a disposable pipette to remove the supernatant. Do not allow the pellet to break free from the bottom of the tube.
- Add 500 µL of cold 70% EtOH (–20 °C) and carefully swirl around the bottom of the tube. Do not vortex (pellet should remain undisturbed).
- Centrifuge for 20 min at 4 °C using the RPM setting from step 2.
- Use a disposable pipette to remove the supernatant.
- If desired, perform a second EtOH wash (steps 4-6).
- Dry by air, lyophilization, or centrifugal evaporation.
The oligonucleotide is now conjugated to the modification and may be dissolved in water or buffer for use in the intended application.
Additional Notes
Preparation of 50 mL of 0.091 M NaB Buffer
- Dissolve 1.735 g of NaB in approximately 45 mL of Milli-Q H2O.
- Allow the powder to fully dissolve prior to adjusting the pH to 8.5 with HCl. Lower the pH by adding HCl in 50 μL portions. Vortex the solution thoroughly and then pipette approximately 25 μL onto a pH strip. Do not dip the pH strip directly into the solution.
- Add Milli-Q H2O until the solution is 50 mL. Vortex the solution again.
- Aliquot 1 mL of the solution into 2 mL tubes until depletion. Store tubes at –20 °C to minimize pH change.
Other non-amine buffers may be substituted for NaB as long as the pH is no higher than 8.5 (a higher pH will hydrolyze the NHS-ester too quickly).
Preparation of NHS-Ester Modifications
NHS-ester modifications come as anhydrous reagents supplied in bottles with airtight bottle-cap seals. Keep moisture away from NHS-esters prior to addition to the amino-labeled oligonucleotide buffer solution as they hydrolyze quickly. Use anhydrous DMSO or another solvent.
- An NHS-ester modification should be stored at –20 °C. Ensure it is completely thawed at room temperature prior to opening the bottle (do not use heat).
- Attach a 20-gauge needle to the tip of a 1 mL syringe. Use this syringe to extract the desired volume of DMSO (use 100 µL per mg of NHS-ester modification solid; depending on the modification, additional DMSO may be necessary). Use the “balloon” technique to maintain the DMSO stock bottle under nitrogen pressure and thereby keep moisture out.
- Add the anhydrous DMSO to the NHS-ester modification bottle. Dissolve the powder by gently pipetting the DMSO up and down while washing the sides of the vial. Gently vortex the bottle to ensure complete dissolution.
- Use the modification right away (storage is not recommended).
To continue reading please sign in or create an account.
Don't Have An Account?