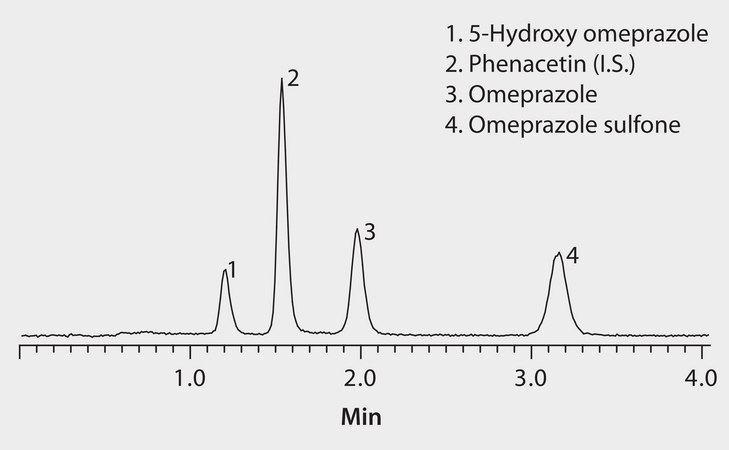
LC/MS Analysis of Omeprazole and Metabolites on Ascentis® Express RP Amide (pH 3.4)
CONDITIONS
column
Ascentis Express RP-Amide, 5 cm x 2.1 mm I.D., 2.7 μm particles (53911-U)
mobile phase
[A] 10 mM ammonium formate in water, pH 3.4; [B] 10 mM ammonium formate in methanol, pH 3.4; (65:35, A:B)
flow rate
0.3 mL/min
column temp.
35 °C
detector
ESI(+), m/z 50-400
injection
1 μL
sample
1 mg/L in mobile phase
Description
Analysis Note
This application demonstrates the suitability of the Ascentis Express RP Amide for the analysis of omeprazole metabolites.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany