HPLC Analysis of Nucleosides on Ascentis® Phenyl
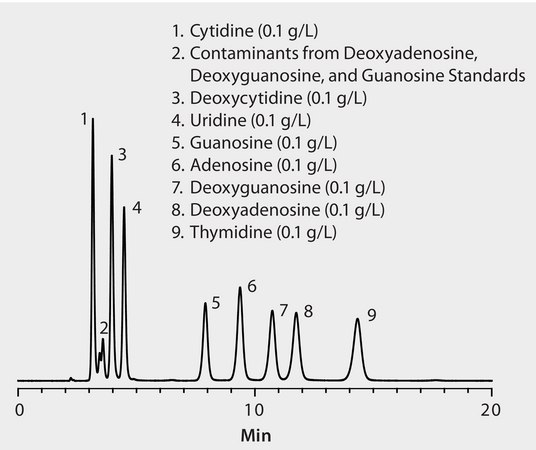
Materials
related product
Product No.
Description
Pricing
CONDITIONS
column
Ascentis Phenyl, 15 cm x 2.1 mm I.D., 5 μm particles (581613-U)
mobile phase
10 mM ammonium formate (pH 3.0 with formic acid)
flow rate
0.2 mL/min
column temp.
35 °C
detector
UV, 270 nm; 750 psi back pressure regulator on outlet of flow cell
injection
1 μL
sample
as indicated, in mobile phase
Description
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany