Lateral Flow Assay Development Services
PLAN YOUR LATERAL FLOW ASSAY DEVELOPMENT FOR SUCCESS
Developing a precise, accurate, and reliable assay is never easy. From raw material sourcing, to technical considerations, to optimization and ultimate scale-up, a development team counters challenges that strain resources and delay timetables. Savvy developers recognize that assay development and optimization move smoother when optimal raw materials and expert support are identified early in the process.

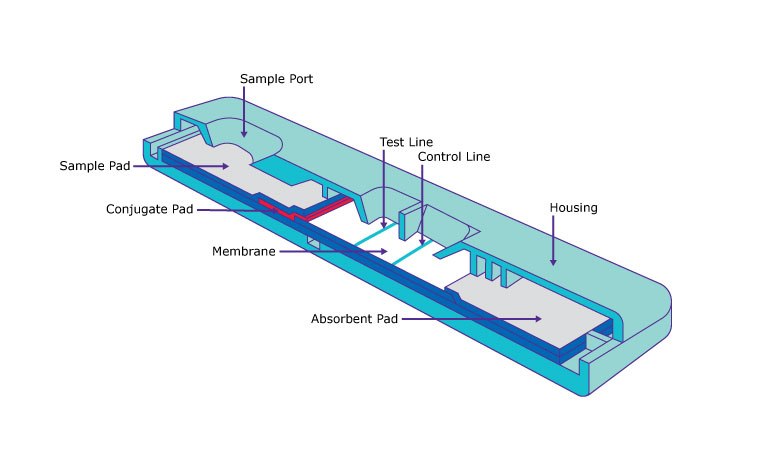
Benefits of Working with a Lateral Flow Membrane Expert
- Utilize vast knowledge of assay raw material market and kit component interactions for sourcing products
- Leverage expert lateral flow assay R&D scientists with dedicated laboratories for troubleshooting
- Avoid common IVD assay development pitfalls and accelerate commercialization
- Reduce time and supplies in optimization steps
- Work with a global partner with robust quality systems
- Expert support can increase confidence in response to regulatory challenges
We are proud to partner with the world’s largest diagnostics manufacturers as well as helping new diagnostics companies navigate compliance requirements and launch the products that will transform healthcare.
Related Resources
- White Paper: Overcoming the Challenges that Delay Development of Your Lateral Flow Assay
This white paper explains overcoming the challenges that delay development of your lateral flow assay and how fast-access support services can get your assay launched sooner.
- Infographic: How Expertise in Design Considerations Affect Cost and Performance
A detailed Infographic illustrating how expertise in design considerations affect cost and performance for assay development services.
- Brochure: Contract Development Services to Accelerate Your Assay Development
Lateral Flow Assay Development Services brochure explains our capabilities on our comprehensive support for accelerating your assay development.
- Application Note: Comparative Analysis of Different EDC and Sulfo‑NHS Activation Reagents
This study was done to demonstrate that Merck EDC and Sulfo-NHS activation reagents (E7750 and 56485) are equivalent to the same Competitor A reagents when used in a two-step covalent coupling bioconjugation procedure.
- White Paper: Optimization & Comparison of Four Different Gold Colloid Products for use in Lateral Flow
4 Gold Colloid Products from different vendors were evaluated and compared in passive conjugation applications for use in a lateral flow assays.
- Application Note: Comparison of Two Casein Grades as Blocking Agents in a hCG Lateral Flow Assay
This study looked at the impact of using two different casein grades in conjugate buffer preparations for a hCG LFA. Buffer preparation times and manipulations were compared, and the results and findings are exhibited in this paper.
- Quality Management Systems
Continuous improvement in systems, processes, and products is essential for maintaining high-quality standards.
- Brochure: IVD Lateral Flow Assay Development
In vitro diagnostic assays encompass various technologies such as PCR, ELISA, CLIA, lateral flow, cytology, and immunohistochemistry, and their development requires expertise in biology, chemistry, physics, and engineering, with considerations of scale-up, quality management, and potential outsourcing for cost-effectiveness and regulatory compliance.
- Rapid Lateral Flow Test Guide
Lateral Flow products and services.
To continue reading please sign in or create an account.
Don't Have An Account?