Protein Stabilizers

Sterile liquid applications demand excipients of the highest quality, purity, and reliability. Our comprehensive portfolio of high-quality pharmaceutical excipients offers everything you need for your medicinal drug product, backed by the regulatory and quality support of the Emprove® Program to streamline approval preparation and accelerate your processes. When your goal is a successful journey to market for small- or large-volume parenteral applications, you can count on our industry know-how, regulatory expertise, and comprehensive documentation to help you go forward with confidence.
High-quality Protein Stabilizers for Biomolecules
Specifically developed for high-risk applications, our portfolio of high-quality sugars, polyols, amino acids, and surfactants simplifies selection when you face critical challenges such as preventing aggregation during manufacturing. Benefits include:
- Low endotoxin and microbial limits
- Elemental Impurity Information according to ICH Q3D
- Emprove® Program and documentation supporting risk assessment
- Emprove® Expert GMP-grade products for high-risk applications
Products
Related Product Resources
- Pharma’s Almanac: Accelerating Biologics Development with High-Quality Protein Stabilization Excipients
About protein instability and aggregation in biologic drug formulation, and how to overcome these challenges.
- Article: How to Prevent Protein Aggregation Using Stabilizers and Surfactants
Read about critical factors in long-term stability of proteins, and how different stabilizers protect against mechanical and thermal stress.
- Article: Low-in-Nanoparticulate-Impurities Sucrose for Biopharmaceutical Formulations
Read about a purification process resulting in low-NPI sucrose to achieve more stable protein formulations.
- Poster: Application of Excipients in Downstream Processing
Learn about the benefits on protein stability, chromatographic performance and viral inactivation.
- Whitepaper: Protecting Protein Stability with a Novel Grade of Sucrose
Get insights on NPIs found in commercially available sucrose, their origin and impact on protein stability.
- Whitepaper: Use of Excipients in Downstream Processing to Improve Protein Purification
Read about the effects of excipients on purification performance in Protein A chromatography and protein stability in virus inactivation.
- Whitepaper: Optimizing Poloxamer 188 for Use in Liquid Protein Formulation and Cell Culture Applications
Dive into stabilization mechanisms of poloxamer 188 and how to use attributes such as molecular weight and hydrophobicity to select the best option for liquid formulation and cell culture applications.
- Flyer: High-Quality Stabilizers for Your Biomolecules
Browse the flyer showcasing key features of our stabilizer portfolio.
- Formulation Product Finder
Quickly sort our excipients and API portfolio by dosage form, application, and many other parameters.
- Biopharmaceutical Application Guide
Explore products and services for mAb, vaccines, microbial, ADC, and plasma processes.
- Risk Mitigation Tool
Get guidance through the challenges and quality requirements of your bio-manufacturing process.
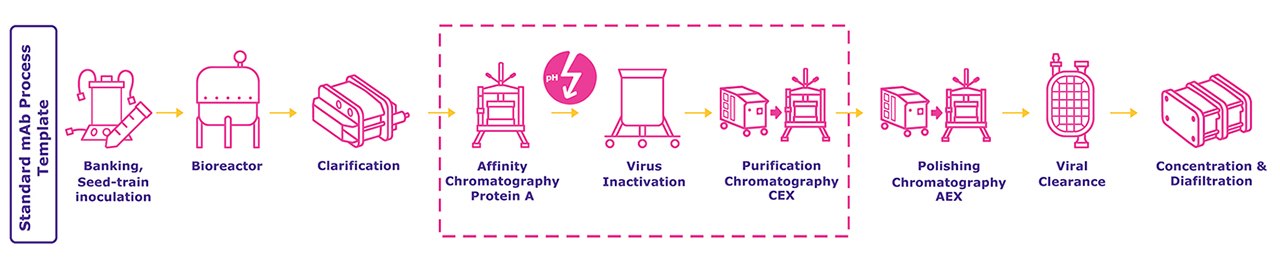
Do you want to increase your efficiency during your Downstream Process?
The production of antibodies and Fc-fusion proteins involves several downstream processing unit operations. The widely used purification template with Protein A chromatography, virus inactivation at low pH, and subsequent ion exchange chromatography steps is mostly able to remove impurities like aggregates, host-cell proteins, and viruses, which could affect the safety and efficacy of the product.
The low pH elution during Protein A chromatography, as well as during virus inactivation may induce aggregation. Preventing protein aggregation during these unit operations instead of removing the multimeric forms during subsequent polishing steps would be a more efficient strategy. Curious to learn more how the aggregation levels in the final product formulation can be minimized by excipients? Click on below image to check out our new Poster “Application of Excipients in Downstream Processing”.
To continue reading please sign in or create an account.
Don't Have An Account?