Hazard Communication & Chemical Regulations
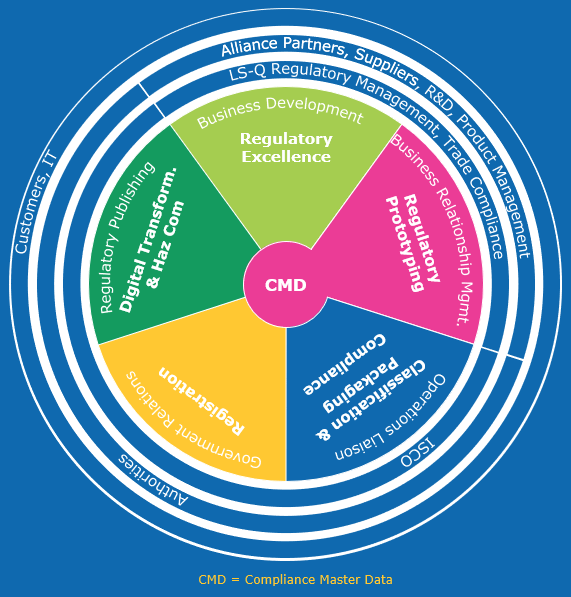
The Hazard Communication and Chemical Regulations department is responsible for implementing global regulatory requirements for placing compliant chemicals on the market and for their safe use based on a proper hazard communication.
Digital Transformation and Hazard Communication
In line with our corporate commitment to Responsible Care and as a trusted partner of our customers, we provide up-to-date, compliant and high-quality hazard information in the areas of Safety Data Sheets, Product Labels, Apps and Websites, thus ensuring the global marketability of our products.
Global Chemical Registrations
As the Regulatory Affairs Department for advanced chemicals programs like REACH, TSCA and MEP Order 7 we enable marketability by taking care of the registration, notification and authorization activities of our comprehensive and innovative Life Science portfolio. By complying with the chemicals regulations in our target markets we deliver the License to Operate. Due to our well recognized expertise and as part of our Government Relations mandate we are advocating several aspects of chemical regulations for our unique Life Science business by closely interacting with the respective authorities and industry associations.
Classification and Packaging Compliance
We are addressing global aspects of Classification and Labeling of our chemicals incl. Dangerous Goods. Therefore, we substantially drive the global implementation of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) within Merck, facing many specialties based on our unique and outstanding Life Science portfolio and services.
Regulatory Excellence
To improve our internal regulatory processes, systems and services for our customers, continuous improvement is part of our DNA. Therefore, dedicated experts in Lean & Six Sigma as well as project management systems are challenging our established processes and systems to enable future growth and additional alliances for our Life Science businesses.
Regulatory Prototyping
Business Relationship of Regulatory functions is key for a sustainable and innovation driven success. Subsequently we conceptualize new regulatory processes and services for innovative products, closely collaborating with Trade Compliance, Regulatory Management and Master Data teams. Furthermore, we take care of Compliance Master Data as the fundament of sound regulatory work.
Digital Transformation and Hazard Communication
The Digital Transformation and Hazard Communication team takes care of Regulatory Publishing comprising classical Hazard Communication (like Label Management and SDS Authoring) and new customer focusing data services (like mobile apps). This globally enables innovation in regulated markets through smart solutions and by driving digitalization.
Furthermore, the reliable handling of data and information and a strong focus on IT systems and processes enables the high quality of our regulatory output. To provide our global customer community a growing product portfolio with best regulatory support (e.g., through high quality Safety Data Sheets or enabling the delivery of our products within 24 hours), it is our ambition and strong belief that:
- Everything that can be digitalized will be digitalized
- Everything that can be connected will be connected
- Everything that can be automated will be automated
Therefore, we are continuously driving projects and developing tailor-made systems to fulfill our stringent quality requirements.
Classification & Packaging Compliance
As part of our Operations Liaison we are working closely at the interface between regulatory and our integrated Supply Chain Operations regarding Classification changes and Packaging of our Products. We have end-to-end responsibility for processing of regulatory product data to ensure overall compliance and safe handling. Our experts in Classification and Labeling of our chemicals as well as dangerous goods classification ensures and maintain the high standard of our hazard communication.
It is our overall aim to:
- Protect the health and safety of our customers, employees and business partners
- Avoid any environmental risks when handling our products
Bundled within our Classification & Packaging Compliance department are all activities around:
- New Product Introduction
- Regulatory Toxicology
- Global Dangerous Goods Management
- Global Packaging Compliance
- Process Improvement
Learn more about:
- Product Labels (PDF)
- Globally Harmonized System (GHS) (PDF)
- Dangerous Goods (PDF)
- Regulatory Toxicology (PDF)
- Quality & Safety App
- My M Safety App
Global Chemical Registration
Fulfilling the regulatory requirements and conducting registrations, notifications or authorizations where required, is the license to operate for our Life Science business.
More than 300,000 chemical identities are part of our Life Science business. Ensuring that all of them fulfill the regulatory requirements of advanced chemicals programs like REACH, TSCA, MEP Order 7 is the job of the Global Chemicals Registration team. One of the daily challenges: meeting constantly changing chemicals requirements in the regulatory environment.
As a globally leading company in Life Science, we take all necessary regulatory actions to expand the supply of our products to our worldwide customer community. The focus in this regard are known highly regulated markets, like the EU controlled by the European Chemical Regulation REACH. Since REACH serves as the blueprint for new regulations within the USA, China, South Korea, Turkey, or the Eurasian Economic Union (EAEU), we can benefit from our comprehensive experience and the global setup to create added value for our customers and ensure compliant products.
In addition, further regulatory programs are also in focus since they become more and more important for market entry. Therefore, we believe it is key to be present in our core markets and assess the impact of new regulations on the Life Science business and set up the necessary tools and processes.
Learn more about the European Chemical Regulation REACH.
Regulatory Excellence
The Regulatory Excellence team is providing services on project management and coordination across the regulatory and compliance functions of our life science business. In the area of premier partnerships, the team is establishing a direct communication between our regulatory departments and the customers counterpart. Regulatory Excellence drives the digital solutions and is responsible to foster a data security concept on daily document and data handling, also enabling a secure data transfer with a restricted use on a need to know principle. Furthermore, the Lean Six Sigma methodology and Kaizen Event approach is fostered to drive efficiency on regulatory and cross-functional processes.
To continue reading please sign in or create an account.
Don't Have An Account?