The Science Behind mRNA and Delivery Shuttles
September 22, 2023 | 6 min
Designing, building and launching a space shuttle is no casual undertaking. It takes years — often decades —of meticulous planning and testing to ensure that the astronaut team will make it to their destination safely. It’s difficult work that requires a diverse range of expertise.
Merck is not expanding its footprint into designing rockets, yet efforts to deliver mRNA — or messenger RNA — to specific locations in the human body have some people thinking about astronauts and space shuttles.
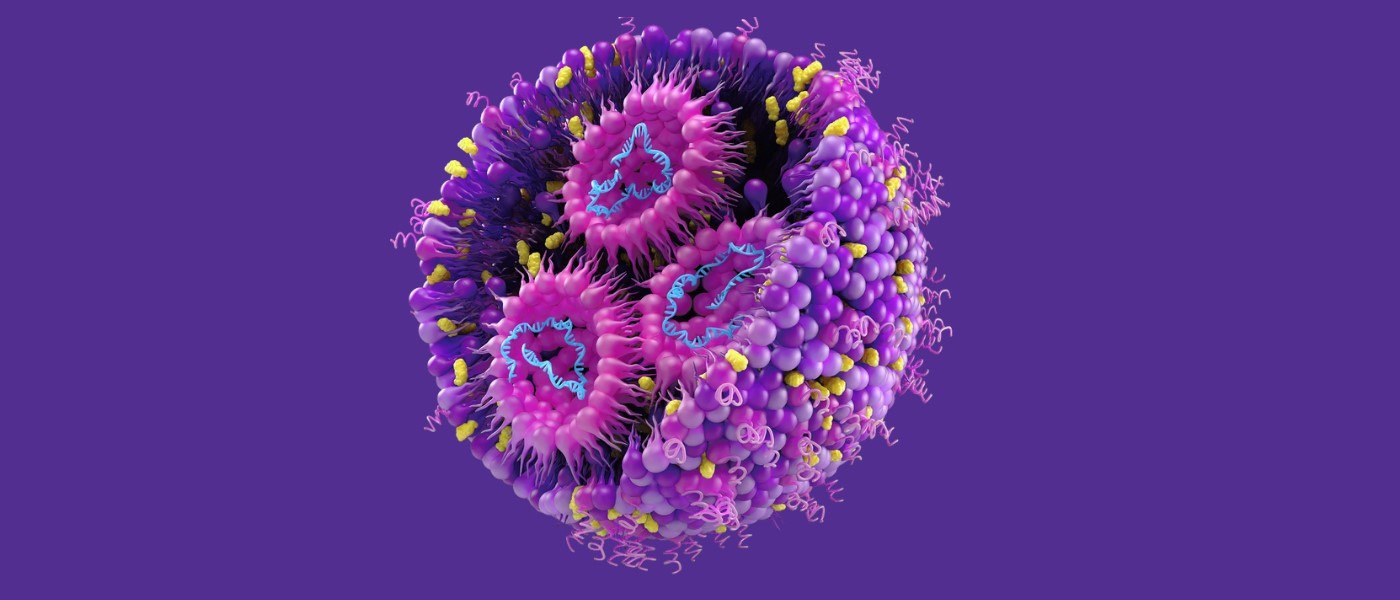
If mRNA is the astronaut, the space shuttle is a lipid nanoparticle (LNP). Astronauts are remembered, but space shuttles rarely are. The same goes for mRNA and LNPs, respectively. LNPs are a type of nanodelivery vehicle — yes, that’s the technical name, and yes, they’re very small objects that deliver even smaller objects.
mRNA vaccines and therapies work by mimicking normal biological processes. In essence, the most effective defense against diseases might already lie within us. The key is to harness that defense. But how?
Every moment, strands of mRNA deliver detailed instructions to tiny “factories” within cells. These factories make proteins, which power every process in the human body. If these instructions get changed, re-routed or destroyed, functions in the cell can go awry, which can lead to illness and disease.
If scientists can correct faulty instructions or send new instructions to specific cells, then these factories could make proteins that prevent or fight off viruses or diseases.
The tailored instructions — or the altered mRNA sequences — just need to make it to the protein factories. But making it to the correct location is no small feat. For all its promise, mRNA itself is incredibly fragile. Sending an astronaut into space without protection isn’t an option, and neither is sending mRNA into the bloodstream. Enter space shuttl-- —err, LNPs.
Scientists — near and far — had worked on the technology for years, but the emergence of COVID-19 rapidly accelerated efforts. Merck started manufacturing lipids decades prior to the emergence of COVID-19 and, more recently, expanded the scope of work to include mRNA and LNPs, through strategic acquisitions.
The promise of mRNA is wide-reaching. Numerous mRNA vaccines are currently in development, including those to ward off human immunodeficiency virus (HIV), flu and malaria.1 And while mRNA vaccines took center stage after COVID-19, the implications span far beyond just vaccines. mRNA-based therapeutics hold tremendous potential to not only treat but cure diseases, including cancer, heart disease, muscular dystrophy and more.2,3,4 If the cellular factory receives the right mRNA instructions to produce replacements for missing or faulty proteins, patients could see improvements to their quality of life.
“mRNA isn’t the answer to everything, but it’s the answer to most things,” says Dr. Aditi Mehta, head of mRNA process and delivery at Merck.
Worldwide, Merck teams cover a wide array of technical expertise — from early foundational research to the final steps before therapeutics ship out. These teams are working to improve processes in labs and on production floors. They’re looking for better ways to make mRNA and LNPs, develop custom lipids for customers quicker and scale up production to meet rising global demands.
Meet People Behind the Science
Before custom LNPs are finalized, there are numerous LNPs that don’t make the cut

Eleni Samaridou, Ph.D.
Darmstadt, Germany
Mission: Work closely with customers to develop and optimize LNP formulations to ensure their therapeutics will provide the best possible treatments to patients across the globe.
Before there are LNPs, there are individual lipids

Tobias Haag, Ph.D.
Schaffhausen, Switzerland
Mission: Design and make large quantities of specialized lipids to ensure LNPs will go to the correct location and keep mRNA protected en route.
Get to know Haag
Before a process is optimized, there are many, many, tweaks

Aditi Mehta, Ph.D.
Darmstadt, Germany
Mission: Identify new ingredients and optimize workflows to improve the process of developing mRNA and LNPs.
Get to know Mehta
Before there are large quantities of mRNA vaccines or therapeutics, there are very small quantities

Mahesh Karwa, Ph.D.
Indianapolis, USA
Mission: Develop and scale up processes and production to systematically bring together final LNP product formulations.
Get to know Karwa
Before the time from idea to final RNA therapeutic or vaccine can shrink, scientists need to formulate viable LNPs

Kahina Lang, PharmD. & MSc
Darmstadt, Germany
Mission: Create a linker library and digitally enabled LNP formulations to reduce the time it takes for scientists to create mRNA therapeutics for patients.
Get to know Lang
About mRNA-Related Offerings
As part of our Millipore® Contract Testing, Development and Manufacturing Organization (CTDMO) Services offering, we pave the way for robust, integrated and consistent processes along all stages —from pre-clinical to commercialization. Bringing together our capabilities, technical expertise and regulatory know-how, we enable our clients to achieve their goals — getting mRNA-based vaccines and therapeutics on the fast track and changing the life of patients.
Citations
To continue reading please sign in or create an account.
Don't Have An Account?