Pyrogen Testing Methods
Pyrogen testing determines the quantity of pyrogens in parenteral pharmaceutical or biopharmaceutical products and is regulated by several standards from organizations such as the Food and Drug Administration (FDA), United States Pharmacopeia (USP), or European Pharmacopeia (EP). The sterility of a product does not imply that it is free of pyrogens. Therefore, drugs that are purported to be sterile must also be tested for pyrogens to prevent febrile reactions in patients.
Pyrogen contamination can occur during production or the administration of pharmaceuticals, biotherapeutics, and medical devices, but the presence of pyrogens can also be an inherent characteristic of the product, such as adjuvants in vaccines or synthetic lipopeptides.
Featured Categories
Ensure safety: Pyrogen testing of pharmaceuticals with the Monocyte Activation Test (MAT) to detect endotoxin, non-endotoxin pyrogens.
Laboratory instruments and consumables for sterility testing in microbiological quality control: Sterility test media, pumps, hardware and accessories.
Speed up your time-to-result with rapid microbial testing using our complete membrane filtration systems; used in bioburden testing, food and beverage analysis, and cannabis microbiology testing.
What is a Pyrogen?
A pyrogen is a substance that causes a rise in temperature (fever reaction) in a human or animal through the activation of the innate immune system. They constitute a heterogeneous group of contaminants comprising microbial and non-microbial substances. Pyrogens can be classified into two groups: endotoxins and non-endotoxin pyrogens (NEPs).
Endotoxins are substances found in Gram-negative bacteria. Non-endotoxin pyrogens are other microbial substances, including those derived from Gram-positive bacteria or viruses and pyrogens originating from yeasts and fungi. Non-microbial pyrogenic substances can also originate from rubber particles, microscopic plastic particles, or metal compounds in elastomers.
Several test methods are available for the detection of pyrogens. They can be classified based on the type of contaminant they detect, and the need for animal materials to perform the test as described in the below table:
Rabbit Pyrogen Testing
The rabbit pyrogen test (RPT) involves measuring the rise in temperature of rabbits following the intravenous injection of the tested product. The RPT gives qualitative results and the sensitivity is quite low. The robustness of the test is also limited, due to development of pyrogen tolerance in rabbits after repeated injections, or stress from the rabbits when performing the assay.
Note: Rabbit Pyrogen Testing (RPT) is now forbidden in Europe, and the Monocyte Activation Test (MAT) is the method of choice for detecting both endotoxin and non-endotoxin pyrogens.
Monocyte Activation Test
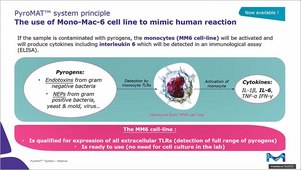
The Monocyte Activation Test (MAT) is an alternative to animal-based methods for the detection of both endotoxin and non-endotoxin pyrogens. The monocyte activation test mimics the human immune reaction by incubating monocytes with the tested sample. If pyrogens are present, monocytes are activated and produce inflammatory molecules, cytokines, responsible for the febrile reaction. The cytokines are then detected using an immunological assay (ELISA) involving specific antibodies and an enzymatic color reaction.
Note: As of July 2025, the European Pharmacopoeia (Ph. Eur.) Commission recommends using the Monocyte Activation Test (MAT) as the preferred method for pyrogen testing. The Rabbit Pyrogen Test (RPT) is now banned in Europe.
Read the article and discuss further with our Experts to move to the MAT detecting endotoxin and non-endotoxin pyrogens and to follow the regulatory trend.
Bacterial Endotoxin Testing (LAL test)
The most common method for endotoxin testing is the Limulus amoebocyte lysate test (LAL test); an assay based on the lysate of amoebocytes from the horseshoe crab blood. The lysate from horseshoe crab blood cells naturally reacts with bacterial endotoxins in a coagulation reaction. This method has a high sensitivity for the quantification of endotoxins, but it does not detect non-endotoxin pyrogens.
Recombinant Factor C (rFC) Testing
The recombinant Factor C is a genetically engineered protein normally found in the Limulus amebocyte lysate cascade. In this test, Factor C reacts with endotoxin and couples with a marker to produce a quantifiable, fluorescent end product. The recombinant Factor C test uses the same principle as the LAL test, but without the need for animal-derived material.
Visit our document search for data sheets, certificates and technical documentation.
- FAQs on bacterial endotoxin contamination, details on endotoxin testing using the LAL assay, and tips to avoid contamination in cell cultures.
- See All (1)
Find More Articles and Protocols
Related Webinars
In this webinar, we discuss how monocyte activation tests performed with the PyroMAT® System detect endotoxin and non-endotoxin pyrogens.
Learn how our PyroMAT® System provides a robust solution for in vitro Pyrogen Test in pharma with a ready-to use kit.
In this lecture, you will learn how to test for pyrogen (including non-endotoxin pyrogens) in your pharmaceutical samples, what are the existing methods in order to have a controlled process.
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?