Steritest® NEO Membrane Filtration Sterility Test
Latest Steritest® NEO Features
Sterility testing is one of the most crucial steps in pharmaceutical product release. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the USP 71, Ph. Eur 2.6.1, and JP 4.06.

The test is particularly suitable for samples containing preservative, bacteriostatic, or fungistatic compounds, which inhibit the microbial growth of potential contaminants. With membrane filtration, microorganisms are retained by a 0.45 µm pore size filter, and all inhibiting compounds are rinsed using a suitable rinse solution. Large volumes and oily products dissolved in emulsifying agents can also be tested by the membrane filtration method, increasing sensitivity and reducing the volume of culture media. Appropriate culture media, selected based upon their ability to support the growth of anaerobic and aerobic microorganisms, are then transferred to the membrane filters. A 14-day incubation period is required to obtain final test results by checking turbidity.
The 4th generation of Steritest® devices is enriching with some additional elements contributing to significantly improving workflow safety, reliability, and convenience. Our sterility test consumables ensure that pharmaceutical products are never exposed to the environment during the testing process. Filtering, rinsing, media transferring, and incubating are all conducted within the Steritest® NEO closed system. There is no need to open the containers or manipulate the membrane at any time, thereby greatly reducing the risk of adventitious contamination resulting in false positives.
When used with the Steritest® Symbio pump and accessories, and Steritest® culture media and rinsing fluids, the Steritest® NEO devices offer the highest levels of quality and reliability and a fully regulatory compliant testing process.
Section Overview
- Latest Steritest® NEO Features
- Meet The Sterility Testing Industry Benchmark
- Sterility Testing Solutions for Products Without Antimicrobial Agents and Medical Devices
- Sterility Testing Solutions for Antibiotics, Products with Antimicrobial Agents and Medical Devices
- Sterility Testing Solution for Products Dissolved in Solvents Requiring Increased Chemical Compatibility
- Sterility Testing Accessories for Liquid Transfer and Dilution
- Sterility Test Sampling Solutions
- Complete Sterility Testing Solutions for Complete Confidence
Latest Steritest® NEO Features
The 4th generation of Steritest® devices with additional elements simplifies every aspect of testing, from handling to traceability to the reliability, all within a closed concept system. The ease and convenience enable you to increase productivity while maintaining the highest levels of quality.
Watch the video to discover all the new improvements.
New features of Steritest® NEO device is created to improve your workflow safety, reliability, and convenience.
Evolution of Safety

The new Velax® cutting clamp allows a safe and clean cut of the tubes

Pre-installed colored clamps prevent any media transfer errors

Redesigned needle guard with grips

Newly designed needle protector with ridges
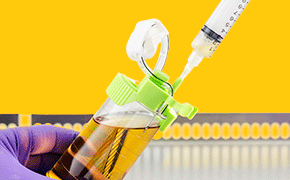
The new needle guide allows safe and easy access to the sample anytime
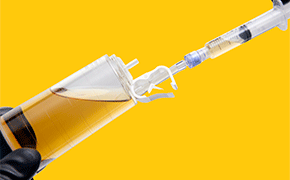
The new needle-free valve is a safe solution for planned sampling or injection
Evolution of Reliability

Clear packaging identification with newly designed color-coded label based on the canister

Peel-off label for improved traceability

Smart Label with 2D data matrix barcode to retrieve product data for easy processing

25 mL volume graduation marks on the canisters
"Coloured clamps ensure ease of tracking tubing to canisters and make it easier to differentiate between media canisters, reducing the chance of wrong incubation.
The new tear bags are easy to open."
- Courtney Smith, Lead Technician - Microbiology Stockton Quality Control Laboratory - University Hospital of North Tees - United Kingdom
Meet the sterility testing industry benchmark
- Minimize false positives: closed Steritest® NEO filtration devices reduce the risk of false positive results and avoid costly investigation and possible batch loss. There are no open containers or membrane manipulations, which could increase the risk of contamination.
- Reduce false negatives: Steritest® NEO filtration devices are the right answer to the danger that false negative results pose to patients. Through specific membranes, unique sealing technology, and optimized device design, the unit allows the efficient elimination of bacteriostatic, fungistatic, or bactericidal agents.
- Easy identification with color coding and optimized traceability.
- Quality check: each Steritest® NEO device is subjected to rigorous in-process and releases quality checks, including 100% membrane and canister integrity tests as well as intense physical and microbiological testing. The detailed certificates of quality are available for download from our website
Get some insights in our high quality production of Steritest® NEO devices in our Center of Excellence, France.

The “Blue Base” Steritest® NEO devices offer the ultimate flexibility. Perfect for use with most pharmaceutical drugs that do not have antimicrobial activity, our mixed esters of cellulose HA membrane allow fast flow rates for optimum throughput performance and reduction in testing time. A range of ergonomic needles is available to meet specific drug packaging requirements as well as simplify handling for gloved operators.
Liquids in ampoules and collapsible bags
Liquids in large vials
Liquids in small vials
Medical devices and collapsible bags
Liquids in syringes
Soluble powders in ampoules
Liquids in plastic containers
Soluble powders in vials

The “Red Base” Steritest® NEO devices, ideally suited for antibiotic sample testing, incorporate our Durapore® (PVDF) HV membrane, offering broad chemical compatibility and low binding properties. The chemical composition of the extremely thin 0.45 μm Durapore® membrane provides low antibiotic binding, which improves rinsing efficiency and reduces the risk of false negative results. The canister design ensures efficient rinsing of residual antimicrobial agents, and the needle and connections minimize the risk of antibiotic residuals
Antibiotics
Powders and super potent antibiotics
Liquids in large vial
Liquids in small vial
Soluble powders in vials
Medical devices and collapsible bags
Recommended accessories

The ‟Green Base” Steritest® NEO devices are suited for viscous products, such as creams and ointments, which are normally diluted in a sterile solvent, such as isopropyl myristate (IPM) to improve filterability. The solvent-resistant nylon canister and the Durapore® (PVDF) HV membrane ensure perfect chemical compatibility with IPM and other solvents. The reinforced base structure and canister connection guarantee an unmatched pressure resistance of the testing device during the whole filtration process. The ‟Green Base” Steritest® NEO devices are the perfect choice for testing solvents, creams, ointments, and veterinary injectables.

Streamline your workflow with our range of sterility testing accessories available for sample preparation and dilution. Steridilutor® NEO devices offers tubing and needle assembly to dissolve powders, or for the transfer of liquids. Steritest® vent needles is used for venting glass vials with rubber septa and rigid plastic vials, or media bottles. The needles can also be used for sterility and growth promotion qualification of media batches.
Sample Preparation and Dilution
Liquid Transfer
Sterile Vent Needles

Throughout the incubation process of Steritest® NEO Devices for compendial sterility testing, it may be necessary to access the container to subculture, reincubate, or identify microorganisms within a turbid medium. It is pivotal to ensure that sterility remains intact and uncompromised throughout these procedures. Mounting a needle guide or a needle-free valve to your canister will help improve safety while performing this task.
Complete Sterility Testing Solutions for Complete Confidence
Discover our full sterility testing portfolio based on over 50 years of experience and expertise. Our large variety of devices and pumps, along with sterile culture media and rinsing fluids can help you to stay compliant, whether you use membrane filtration or direct inoculation methods. Reduce the sterility testing workload and focus on your critical lab activities thanks to our extended Steritest® services portfolio. The experienced application and validation engineers will assist in method development and validation implementation within the QC microbiology laboratory. They will also provide basic and advanced technical training on sterility testing, in person or remotely.
To continue reading please sign in or create an account.
Don't Have An Account?






