Polyethylene Glycol Building Blocks for PEGylation
Circulatory half-life is a key success factor for new drugs. In this respect, PEGylation or PEG-ing—the modification of potential candidates ranging from non-peptidic small molecules to peptides and proteins, antibody fragments, aptamers, and saccharides or oligonucleotides with polyethylene glycol chains—offers numerous advantages.
PEGylation is considered one of the most successful techniques to enhance the therapeutic and biotechnological potential of peptides and proteins.1
- PEGs are non-toxic, non-immunogenic, non-antigenic, highly soluble in water, and FDA approved.2
- The PEGylated conjugates show a decreased degradation by metabolic enzymes and a reduction or elimination of protein immunogenicity as the PEG coating prevents the approach or recognition by proteolytic enzymes and antibodies. (Scheme 1)
- Furthermore, PEGylation may favorably alter drug distribution in the organism.3
- While PEGylation may improve the pharmacokinetic properties of a drug, the drug’s main activity is predominantly preserved.4
Since the first therapeutic PEG-protein conjugate (PEG-adenosine deaminase) had been approved by the FDA in 1990, a large number of new PEGylated drugs was introduced to the market representing a multi-billion dollar business.2 Table 1 summarizes a selection of FDA approved PEG conjugates including therapeutic peptides, proteins, small molecules, oligonucleotides, and antibodies:
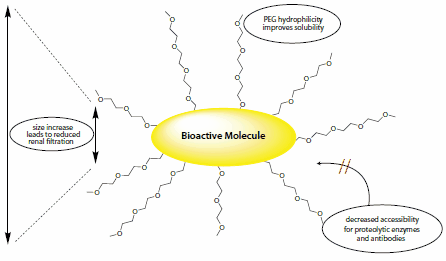
Scheme 1.PEGylation may improve the properties of drug candidates
Drug Discovery Applications for PEGs
More recently, researchers have enforced strategies that utilize releasable PEGs in order to control protein release rates.10 Usually a trigger moiety is introduced between protein and PEG that can be cleaved enzymatically or by aqueous hydrolysis.
Further important applications of functionalized polyethylene glycols apart from drug discovery are:
- Introduction of solubilizing handles in SPPS11
- oluble polymer supports for Peptide Synthesis12
- oluble polymer supports for Organic Synthesis13
- Introduction of hydrophilic amino acids in Peptide Synthesis14
- Preparation of PEG-coated surfaces15
- Linking of macromolecules to surfaces16
- Preparation of PEG-cofactor adducts for biorectors17
Reagents for PEGylation
We are proud to offer a comprehensive portfolio of PEG reagents, with molecular weights up to 20 kDa for efficient PEGylations. Numerous homobifunctionalized, heterobifunctionalized, and mono-methoxy endcapped monofunctionalized PEG’s are available in high quality and monodispersity. We provide polyethylene glycols activated for the most widely used conjugation techniques to primary amines or thiols. The amino groups (e.g. lysine) of proteins and peptides are the preferred choice for PEG conjugation by alkylation or acylation. Alkylation maintains a positively charged amine, whereas acylation yields a neutral amide linkage. Acylation can be realized most conveniently with N-hydroxysuccinimide (NHS) activated carboxylated PEGs. Azide functionalized PEGs allow the application of Staudinger ligation and Huisgen dipolar cycloaddition techniques. The introduction of different protecting groups leads to extremely useful macromolecular cross-linking agents and spacers. All common target moieties can be specifically addressed with the appropriate functionalized PEG linker. Please find a collection of our most recent additions to our portfolio of PEGylation products below, and visit us on the web for a full product listing.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?