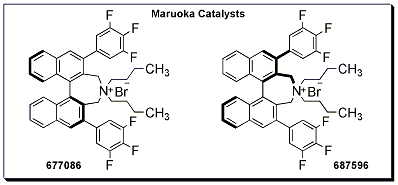
Maruoka Catalysts
Asymmetric phase transfer catalysis using the Maruoka catalysts has proven to be an ideal method for the enantioselective preparation of natural and unnatural α-alkyl and α,α-dialkyl-α-amino acids from glycine derivatives. While various related catalysts have been developed for this type of transformation, 677086 and 687596 are particularly noteworthy because of their structurally simplified skeleton. In addition to their relatively simpler structure, 677086 and 687596 also maintain comparable catalytic activity and the ability to achieve enantioselectivities equivalent to catalysts previously designed by Maruoka and coworkers.1,2,3
Advantages
- Operationally straightforward
- Environmentally friendly
- Relatively mild reaction conditions
- Reactions conducted in aqueous media
- Compatible with scale-up requirements
- Low catalyst loading
Representative Applications
Maruoka and coworkers reported in 2005 another version of their chiral phase transfer catalysts which utilized butyl groups in place of biphenyl or naphthyl groups on the nitrogen, providing a more flexible and more polar catalyst, facilitating the exchange required to take place between the organic and aqueous layers of the biphasic mixture.2
A typical procedure for the biphasic transformation requires the use of an organic solvent (toluene) in combination with an aqueous solution of base, the phase transfer catalyst, the organic halide, and the protected amino acid (usually protected as a benzophenone imine or p-chlorobenzaldehyde imine-Tables 1 and 2). Most reactions proceed with relatively short reaction times at temperatures varying from -20 °C to 0 °C to provide the α-alkyl and α,α-dialkyl-α-amino acids in good overall yield with excellent enantioselectivities.1,2,3
Initially, it was thought that the use of the more economical p-chlorobenzaldehyde imine would be limited to the α-alkyl substituted derivatives because of the lower pKa and concern for racemization of these aldimine Schiff bases; however, it was later divulged that the p-Cl-phenyl protecting group does indeed allow for effective transformation to the monoalkylation derivatives such as those shown in Table 2.3
The method also affords α,α-dialkyl-α-amino acids in good yields with excellent enantioselectivities when alanine-derived Schiff base is used as shown in Scheme 1.
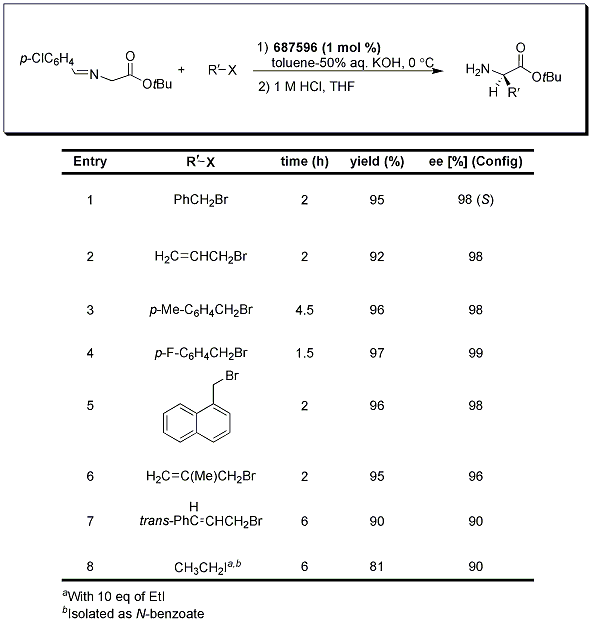
Table 1.Benzophenone imine
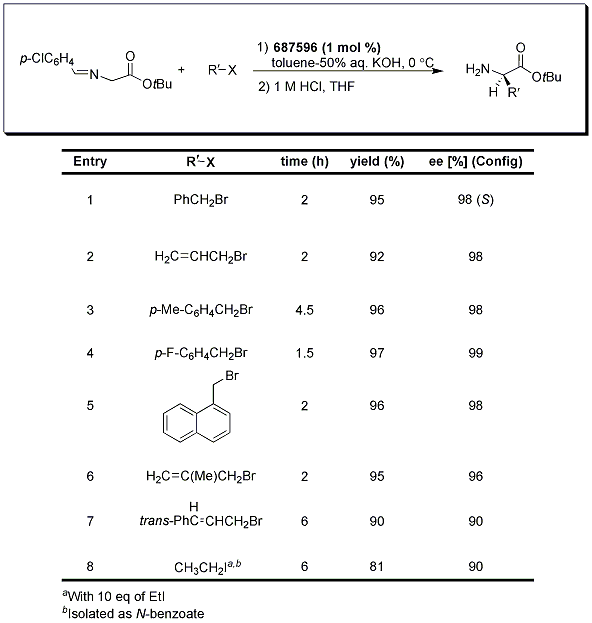
Table 2.p-chlorobenzaldehyde imine
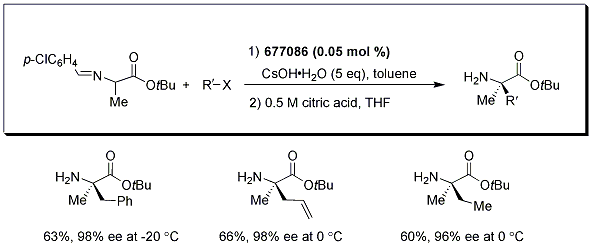
Scheme 1.alanine-derived Schiff base
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?