N-Heterocyclic Carbene (NHC) Ligands & Kit for Organic Transformations
Rapid progress in cross-coupling reactions of unactivated substrates catalyzed by metal complexes has transformed the chemical market-place through introduction of an extensive library of achiral and chiral phosphine ligands. The positive effects of phosphine ligands are exemplified in the numerous reports of highly efficient systems in terms of turnovers and selectivities applied to traditional industrial processes such as hydrogenation. However, the disadvantages in the high cost of producing tertiary (especially chiral) phosphines and their degradative tendency in converting to phosphine oxides have only now been addressed by the rich field of NHC ligands utilized in homogeneous catalysis.1
The advantages of N-heterocyclic carbenes as ancillary ligands are:
- they are stronger s-donors than phosphines, enabling favorable rates of palladium catalyzed oxidative addition of aryl chlorides;
- the strong metal–carbenic bond of the NHC complex favors tight binding kinetics, therefore lessening ligand dissociation;
- the presence of sterically encumbering groups bound to the N-atoms facilitate reductive elimination of the product from palladium; and
- the activity of NHC ligands can be modified by the introduction of electronic directing substituents remotely, as witnessed in the synthesis of benzimidazolidines that contain electronically dissimilar groups on the aromatic backbone.2
A wide range of NHC ligands that exhibit high activities in various important organic transformations when combined with metal pre-catalysts are now commercially available. NHC imidazolidine ligands with sterically encumbering groups such as mesityl, isopropyl, and adamantyl have been used in the Pd-catalyzed cyclization of anilides,3 amination of aryl chlorides,4 arylation with ester enolates to afford a-aryl esters,5 Sonogashira reactions of unactivated alkyl bromides,6 and the ruthenium-catalyzed RCM reaction (Schemes 1–5).7 This last example shows the power of NHC ligands to stabilize the metal active species in solution and thus imparts the ability of the catalyst to effect RCM on highly substituted and electron-poor olefinic reactants to yield tetrasubstituted products. Such pronounced reactivity was the exclusive domain of the Shrock alkylidene catalysts.8 However, use of these imidazolidines leads to an improvement in performance over early ruthenium metathesis catalysts and provides additional stability features versus the Shrock system. Our goal at Sigma-Aldrich is to accelerate cutting-edge research projects in NHC ligand mediated chemistry. To this end, we are the first to offer a ligand kit composed of a diverse set of sterically demanding NHC ligands.
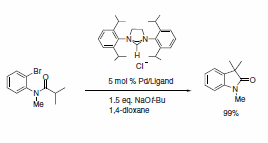
Scheme 1.

Scheme 2.
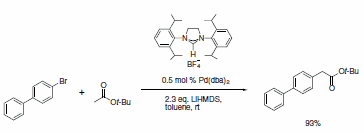
Scheme 3.
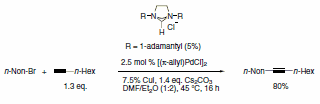
Scheme 4.

Scheme 5.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?