TADDOL
The chiral auxiliaries TADDOLs (α,α,α,α-tetraaryl-1,3-dioxolane-4,5- dimethanols) developed by Seebach's group have found numerous applications in asymmetric synthesis ranging from utilization as stoichiometric chiral reagents or in Lewis acid mediated reactions, to roles in catalytic hydrogenation and stereoregular metathesis polymerization.1
Nucleophilic Addition
The principal field of application of TADDOLs to date is in Ti-catalyzed, enantioselective reactions. Nucleophilic additions of organometallic compounds to aldehydes are depicted in Scheme 1. The preparation of the Ti-TADDOLate complex used in such transformations is critical with respect to reproducibility.1
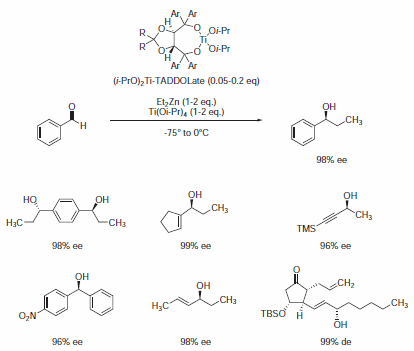
Scheme 1
Enantioselective transfers of allyl groups to aldehydes are most successful using Duthaler's CpTi-TADDOLate complexes 449520 or 449539 (Scheme 2).2
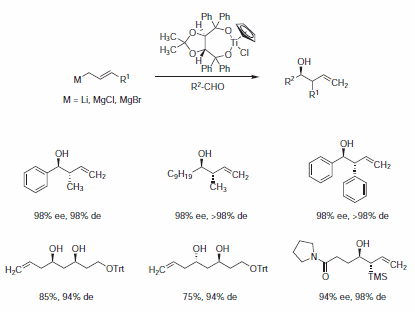
Scheme 2
Enantioselective Transesterification
Several examples of enantioselective transesterifications were reported for ring openings of lactones and cyclic anhydrides using Ti-TADDOLate of 393762 (Scheme 3). The observed selectivity in this desymmetrization step is largely independent of structure. The half esters obtained are readily converted into the corresponding γ-lactones.3
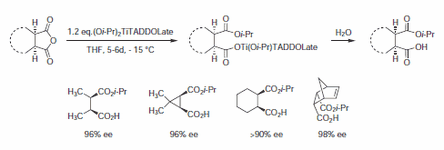
Scheme 3
It is worth mentioning that TADDOL 395242 bearing 1‑naphthyl groups infrequently differs in its reactivity from the other TADDOLs (a dramatic loss in enantioselectivity is observed in Ti-TADDOLate catalyzed addition reactions described previously). This change in reactivity could be explained with the higher steric hindrance around the chiral pocket of the active site.4
Enantioselective Protonation
Nevertheless, surprisingly stereoselective examples using TADDOL 395242 were found in enantioselective protonation reactions (Scheme 4),5 the first example of a catalytic enantioselective fluorination reaction reported by Hintermann and Togni used the Cl2Ti-complex of 395242 (Scheme 5)6 as well as the recent example reported by Rawal using 395242 as a Brønsted acid organocatalyst in a highly enantioselective hetero-Diels-Alder reaction (Scheme 6).7
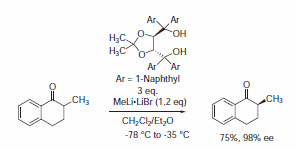
Scheme 4

Scheme 5
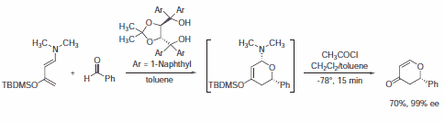
Scheme 6
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?